![]() https://doi.org/10.35845/kmuj.2024.23705 SYSTEMATIC
REVIEW
https://doi.org/10.35845/kmuj.2024.23705 SYSTEMATIC
REVIEW
Long term thalidomide therapy’s efficacy and safety in transfusion-dependent beta thalassemia major patients: a systematic review
Tahira
Atta ![]() 1,
Zakia Subhan
1,
Zakia Subhan ![]() 2,3,
Muhammad Nabi
2,3,
Muhammad Nabi ![]() 3,
Nur-Ul-Ain
3,
Nur-Ul-Ain ![]() 3,
Wajid Ali
3,
Wajid Ali ![]() 3,4
3,4
|
1: Department of Pathology, KMU Institute of Medical Sciences, Kohat, Pakistan 2: Department of Pharmacology, KMU Institute of Medical Sciences, Kohat, Pakistan 3: Institute of Pharmaceutical Sciences, Khyber Medical University, Peshawar, Pakistan 4: Department of Pharmacology, Ayub Medical College, Abbottabad, Pakistan
Email
Contact #: +92-333-5715816
Date Submitted: June 13, 2024 Date Revised: August 27, 2024 Date Accepted: August 30, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Atta T, Subhan Z, Nabi M, Ain NU, Ali W. Long term thalidomide therapy’s efficacy and safety in transfusion-dependent beta thalassemia major patients: a systematic review. Khyber Med Univ J 2024;16(3):255-62. https://doi.org/10.35845/kmuj.2024.23705 |
ABSTRACT
OBJECTIVE: To identify the effectiveness and safety of thalidomide in transfusion-dependent beta Thalassemia Major (TDBTM) patients.
METHODS: A comprehensive search of PubMed, Cochrane Library, and Embase was conducted between January 01, 2014, and April 17, 2024, using terms like “thalidomide”, “thalidomide”, “α- phthalimidoglutarimide”, “thalassemia”, “beta thalassemia”, “transfusion dependent thalassemia” using Boolean or wildcard operators. Studies published in English with an observational or experimental design, including more than 10 TDBTM patients treated with thalidomide for at least 3 months, were included. The review focused on patients of all ages and genders, evaluating the impact of thalidomide on transfusion requirements. The included trials, involved 780 participants (age range=1.5-27.2 years), showing improvements in hemoglobin, fetal hemoglobin (HbF), serum ferritin, spleen size, and quality of life. National Institute of Health tool was used for quality assessment.
RESULTS: After screening 19462records, 18147remained after duplicates were removed. Of these, 18138 were excluded, leaving 09 studies for inclusion in the review. Conducted in Pakistan, China, India and Iraq (2014-2024), the studies included a single-arm trial, a double-blinded RCT, and an open-label RCT and Pre-post enrolling 780 participants. Thalidomide (50-150 mg/day) improved hemoglobin, HbF, serum ferritin, spleen size, and quality of life, with a mean follow-up of 13 months. Thalidomide therapy resulted in transfusion independence in 69.6 % (n= 543) patients. Adverse effects were reported in 41.2 % (n=322) patients. Study quality was rated as good.
CONCLUSION: Thalidomide is a well-tolerated, safe, and effective treatment for TDBTM patients, but these findings require confirmation through well-designed clinical trials.
KEYWORDS: Thalidomide (MeSH); Beta-Thalassemia (MeSH); Transfusion Dependence (Non-MeSH); Anemia (MeSH); Blood Transfusion (MeSH); Erythrocyte Transfusion (MeSH); Thalassemia Major (MeSH); beta-Thalassemia (MeSH); Thalidomide Therapy (Non-MeSH); Transfusion Independence (Non-MeSH); Hemoglobinopathies (MeSH).
INTRODUCTION
Thalassemia is one of the most prevalent hereditary anemias, resulting from a reduced production of globin chains.1 This genetic disorder is common in tropical and subtropical regions, including the Mediterranean, Africa, the Indian subcontinent, and Asia, where the estimated frequency of alpha thalassemia ranges from 12-50% and beta thalassemia from 1-20%. 2, 3 Beta thalassemia is characterized by defective synthesis of beta globin chains, leading to reduced hemoglobin (Hb) production, anemia, and a lower red blood cell count. It can manifest in three forms: beta thalassemia minor, intermedia, and major, and may co-occur with other hemoglobin disorders, such as HbC/beta thalassemia, HbE/beta thalassemia, and HbS/beta thalassemia.4 The genetic mutations responsible for beta thalassemia vary and include point mutations, deletions, and insertions.5 The severity of beta thalassemia varies based on the specific mutations in an individual's DNA. Certain mutations can cause more severe symptoms and complications, whereas others may result in a milder form of the condition. Clinically, Beta thalassemia can be classified as transfusion-dependent thalassemia (TDBTM) and non-transfusion-dependent thalassemia.6
For patients with thalassemia major, the primary treatment consists of regular blood transfusions and iron chelation to manage iron overload. Other therapeutic options include hematopoietic stem cell transplantation (HSCT), gene therapy, and erythroid maturation agents.7-9 Currently, around 100,000 patients worldwide rely on regular blood transfusions.7 However, chronic transfusions elevate the risk of viral infections, acute hemolytic reactions, allergies, and other life-threatening complications. Additionally, they lead to iron overload, which can cause significant damage to multiple organs.10
A promising alternative therapy is thalidomide, an immunomodulatory drug and potent fetal hemoglobin (HbF) inducer. Thalidomide enhances γ-globin gene expression, raising HbF levels and subsequently increasing hemoglobin concentration, which reduces the need for transfusions in thalassemia patients.11 For patients unable to undergo allogeneic stem cell transplantation, thalidomide offers a practical, cost-effective therapeutic option with a relatively low rate of adverse reactions. Furthermore, thalidomide has been proposed as a bridge between gene therapy and transplantation, potentially minimizing the transfusion burden12 However, its safety profile and efficacy in transfusion-dependent thalassemia require systematic evaluation.
Rationale for the review: Although thalidomide has shown promise in reducing transfusion dependence by increasing HbF production, there is a need for a comprehensive evaluation of its effectiveness, safety, and potential side effects in transfusion-dependent beta thalassemia major patients. This systematic review aims to synthesize existing evidence on thalidomide's impact, with the goal of providing clearer guidance for its use in clinical practice. By examining the current data on its therapeutic potential, this review seeks to minimize transfusion-related complications and improve outcomes for TDBTM patients.
This review will contribute to the body of knowledge by offering insights into how thalidomide can be integrated into treatment protocols, addressing both its benefits and limitations, and helping to reduce the overall burden of transfusions in thalassemia care.
METHODS
Data source and search strategy: A comprehensive literature search was conducted using databases such as PubMed, Cochrane Library, and Embase to evaluate the effectiveness and tolerability of thalidomide in beta thalassemia major patients receiving regular blood transfusions. The study included patients of all ages and both genders, with data collected from January 01, 2014 to April 17, 2024. The search terms used were: "thalidomide," "α-thalimidoglutarimide," "thalassemia," "beta thalassemia," and "transfusion-dependent thalassemia," incorporating Boolean operators and wildcards where appropriate. A detailed list of search terms can be found in Appendix 1. The search followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to identify prospective studies.13
Study selection: This systematic review included full-text original articles with an observational or experimental design, published in English between Janua 01, 2014, and April 17, 2024. Selected studies included 10 or more transfusion-dependent beta thalassemia major patients of any age and gender who had been treated with thalidomide for a minimum of 3 months. Studies that focused on non-transfusion-dependent patients, beta thalassemia intermedia, case series, case reports, systematic reviews, or conference abstracts were excluded.
Data extraction and quality assessment: Data were extracted by three independent reviewers (TA, ZS, and WA), who assessed each study for internal validity. After extracting data, the reviewers discussed any potential biases. The following data points were collected after reaching a consensus: author, year of publication, study design, country, sample size, patient age, thalidomide dose, transfusion independence, follow-up duration, genotype, adverse effects, and patient response to therapy. The quality of the included studies was assessed using the National Institutes of Health (NIH) quality assessment tool.14
RESULTS
Study selection: After the initial database search, 19462 records were identified. Once duplicates were removed, 18147records remained. Of these, 17752 were excluded, leaving 395 records for further screening. Among these, 386 studies were excluded for the following reasons: 362 did not meet the inclusion criteria, and 04 were conference papers, 02 were Case Reports, 02 editorials, 02 were letter to editor and 14 review articles. Finally, 09 studies were included in the systematic review. [Figure 1]
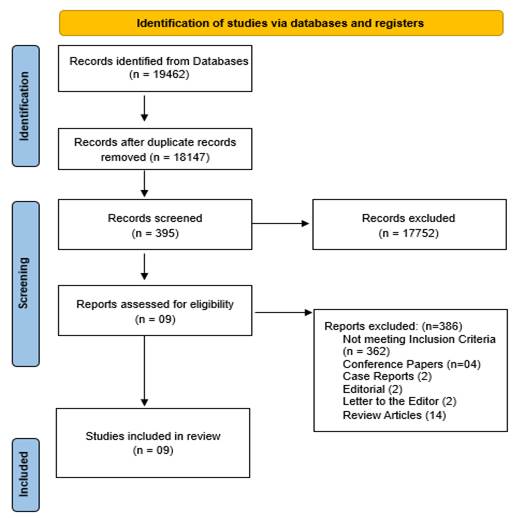
Figure 1: PRISMA 2020 flow diagram for systematic review
Study characteristics
Table I summarizes the general characteristics of the patients included in the studies. These clinical trials were conducted between January 01, 2014, and April 17, 2024, across three countries: Pakistan, China, India and Iraq. Among the studies, one was a single-arm, non-randomized clinical trial, one was a double-blinded randomized controlled trial (RCT), one was an open-label RCT and 06 were pre-post. All trials were conducted prospectively, with full-text publications available in English.11, 15-22 Collectively, the studies enrolled a total of 780 participants, with sample sizes ranging from 14 to 532, including both children and adults (ages 1.5-27.2 years). In three of the studies, patients received blood transfusions exclusively while on thalidomide,11, 15 whereas in one study, additional medications such as clopidogrel, iron chelators, dietary supplements, antihistamines, and hepatoprotective agents were administered alongside thalidomide. One study reported supportive care including adenosine triphosphate for lethargy, fiber rich diet for constipation, vitamin D for arthralgia, vitamin B6 for numbness, anti-allergic agents for rash, and liver protecting agents. Aspirin were prescribed to patients with platelets >500×109 /L to prevent thrombosis.17 Blood transfusions were provided to patients exhibiting hemodynamic instability or those unable to maintain a hemoglobin level of 6 g/dL or above.16
All patients received oral thalidomide, administered in doses ranging from 50 to 150 mg per day. Two of the studies reported both baseline and post-treatment hemoglobin levels, serum ferritin levels, and spleen size, 15, 16 while one study also included quality of life assessments and hemoglobin F (HbF) percentage.11 The studies demonstrated improvements in hemoglobin and HbF concentrations, reductions in serum ferritin and spleen size, and enhancements in quality of life (Table II). Chen J et al.,11 further reported a reduction in serum concentrations of lactate dehydrogenase (LDH), α-hydroxybutyric dehydrogenase, urea, uric acid, glutamyl transpeptidase, adenosine deaminase, globulin, total bilirubin, and indirect bilirubin, with an increase in the albumin/globulin ratio. Ali Z et al.,16 observed increases in red blood cell counts, mean corpuscular hemoglobin, red cell distribution width, and platelet counts, along with decreases in absolute neutrophil count, LDH, and uric acid levels. Yang K et al.,21, 22 reported Increase in Red blood cell count, MCV, MCH, indirect bilirubin and LDH. The follow-up periods across the studies ranged from 3 to 30 months, with the mean follow-up duration being 13 months, during which the effectiveness and safety of thalidomide were evaluated.
Table I: General characteristics of patients
|
Authors |
Design |
Country |
Disease |
Population |
Age (years) |
Sample size (n=780) |
Thalidomide dose |
Transfusion independence |
Follow-up |
Genotype |
|
Ali Z et al., 2023 16 |
Off label clinical trial |
Pakistan |
BTM |
TDBTM |
1.5-26 |
532 |
Max. 70 mg/day |
408 |
30 |
NR |
|
Bhattacharjee U et al., 2023 15 |
RCT |
India |
BTM |
TDBTM |
26.14 ± 4.276 |
14 |
50 mg/day |
NR |
6 |
β0/β0, β0/β+ |
|
Chen J et al., 2021 11 |
RCT |
China |
BTM |
TDBTM |
18.4 ± 5.6 |
50 |
100-150 mg/day |
34 |
3 |
β0/non-β0, non-β0/non-β0, β0/β0, Xmn I genotype, (G/G, A/G), Simultaneous α globin mutation |
|
Li X et al., 2021 17 |
Pre-post |
China |
β-TM |
TDBTM |
10 (5-18) |
77 |
2.5-4 |
51 |
6 |
β0/β+, β+/β+, β0/β0 |
|
Chandra J et al., 2021 18 |
Pre-post |
India |
HbE-β-TM |
TDBTM |
14.7 (12-18) |
37 |
2-4 |
15 |
6 |
B0/ Severe β+, E β-double heterozygous |
|
Yang K et al., 2020 22 |
Pre-post |
China |
β-TM |
TDBTM |
27.7 (NR) |
12 |
50a |
5 |
3 |
NR |
|
Nag A et al., 2020 20 |
Pre-post |
India |
HbE-β-TM |
TDBTM |
20**(NR) |
21 |
50-100a |
15 |
3 |
NR |
|
Yang K et al., 2020 21 |
Pre-post |
China |
β-TM |
TDBTM |
27.2 (15-45) |
23 |
50-100a |
10 |
24 |
β0/β+, β+/β+, β0/β0, β0/-, -/- |
|
Yassin AK 2019 19 |
Pre-post |
Iraq |
β-TM |
TDBTM |
10** (3-43) |
14 |
2-10 |
5 |
8-36 |
NR |
RCT = Randomized clinical trial; BTM = beta thalassemia major; TDBTM = transfusion dependent beta thalassemia; NR = not reported, Max = Maximum; (a): mg/day, (*): Mean age; (**): Median age,
Table II: Response of TDT patients upon thalidomide therapy
|
Authors |
Parameters |
||||
|
Hemoglobin (g/dl) |
Serum Ferritin (ng/ml) |
Spleen size (cm) |
HbF (%) |
QOL (ECOG score) |
|
|
Ali Z et al., 2023 16 |
From 6 to 8 |
From 2195 to 528 |
From 6.4±3.3 to 5.6±3 |
NR |
NR |
|
Bhattacharjee U et al., 2023 15 |
From 8.7±0.99 to 9.2± 1.56 |
From 3309.4±3342.39 to 2587.4±2204.39 |
No change |
NR |
NR |
|
Chen J et al., 2021 11 |
From 7.24±1.6 to 9.1±1.7 |
From 2937.8 to 2732.3 |
NR |
From 10.8 to 55.2 |
0.8 ± 0.7 |
|
Li X et al., 2021 17 |
NR |
NR |
NR |
From 8.87 to 84.2 |
NR |
|
Chandra J et al., 2021 18 |
From 9.45 to 8.89 |
From 1758.9 to 1549.6 |
NR |
From 2.95 to 49.2 |
NR |
|
Yang K et al., 2020 22 |
From 6.1±1.7 to 8.5±1.4 |
NR |
From 10.4±4.5 to 10.7±5.4 |
From 35.6±22.6 to 60.8±18.8 |
NR |
|
Nag A et al., 2020 20 |
from 6.5 (± 1.08) to 7.2 (± 1.74) |
1919 ng/ml to 1404 ng/ml |
From 7.22 ± 1.37 to 7.56 ± 1.14 |
NR |
NR |
|
Yang K et al., 2020 21 |
NR |
NR |
NR |
NR |
NR |
|
Yassin AK 2019 19 |
NR |
NR |
NR |
NR |
NR |
QOL= Quality of life, ECOG= Eastern Cooperative Oncology Group; NR=not reported; TDT = transfusion dependent beta thalassemia
Quality Assessment of the included studies
The quality of the studies included in the systematic review was evaluated using the National Health Institute assessment tool. Following the assessment, all studies were classified as having good quality (Table III).
Table III: National Institute of Health Quality Assessment Tool
|
Criteria |
Ali Z et al., 2023 16 |
Bhattacharjee U et al., 2023 15 |
Chen J et al., 2021 11 |
Li X et al., 2021 17 |
Chandra J et al., 202118 |
Yang K et al., 2020 21 |
Nag A et al., 2020 20 |
Yang K et al., 2020 22 |
Yassin AK 2019 19 |
|
1. Was the research question or objective in this paper clearly stated? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
2. Was the study population clearly specified and defined? |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
|
3. Was the participation rate of eligible persons at least 50%? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
5. Was a sample size justification, power description, or variance and effect estimates provided? |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
10. Was the exposure(s) assessed more than once over time? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
12. Were the outcome assessors blinded to the exposure status of participants? |
No |
No |
Yes |
No |
No |
No |
No |
No |
No |
|
13. Was loss to follow-up after baseline 20% or less? |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
Quality rating |
Good |
Good |
Good |
Good |
Good |
Fair |
Good |
Good |
Fair |
Adverse effects
Table IV presents the adverse events associated with thalidomide use. Most patients experienced mild side effects, including constipation, dizziness, skin rash, abdominal pain, fatigue, fever, sore throat, nausea, and drowsiness. However, some patients developed more severe conditions, such as grade 3 syncope, grade 2 hyperbilirubinemia, and grade 2 transaminitis. A total of 14 patients discontinued treatment due to side effects, including stroke (n=1), cerebrovascular accident (CVA) (n=3), portal vein thrombosis (PVT) (n=2), grade 3 syncope (n=1), grade 2 drowsiness (n=1), and grade 2 headache (n=1), acute kidney injury (AKI) (n=1), neutropenia (n=1), Grade 2 sedation (n=1), seizures (n=1) and extra-medullary hemopoiesis (n=1).
Table IV: Adverse effects
|
Authors (Year) |
Adverse Effects |
||||||||||
|
Ali Z et al., 2023 16 |
Constipation (33;6.2%) |
Neutropenia (6;1.1%) |
Skin rash (5;0.9%) |
Paresthesia (4;0.8%) |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
|
Bhattacharjee U et al., 2023 15 |
Grade 1 somnolence (42.8%) |
Grade 2 somnolence (35.7%) |
Grade 2 presyncope (7.1%) |
grade 3 syncope (7.1%) |
grade 2 headache (14.3%) |
grade 2 hyperbilirubinemia (7.1%) |
grade 2 transaminitis (7.1%) |
grade 1 constipation (7.1%) |
-------- |
-------- |
-------- |
|
Chen J et al., 2021 11 |
Dizziness (20; 40.8%) |
drowsiness (21;42.9%) |
Fatigue (15; 30.6%) |
rash (9; 18.4%) |
Pyrexia (12; 24.5%) |
Sore throat (7; 14.3%) |
Constipation (5; 10.2%) |
Abdominal Pain (n=7; 14.3%) |
Nausea (8; 16.3%) |
Limbs edema (5;10.2%) |
-------- |
|
Li X et al., 2021 17 |
Dizziness/ Lethargy (24; 31.2%) |
Constipation (9; 11.7%) |
Neutropenia (10; 13%) |
Leukocytopenia (7; 9.1%) |
Thrombocytosis (9; 11.7%) |
CVT* (1; 1.3%) |
Seizure* (1; 1.3%) |
Vomiting/ Nausea (9; 11.7%) |
Arthralgia (4; 5.2%) |
Edema (3; 3.9%) |
peripheral neuropathy / headache (02; 2.6%) |
|
Chandra J et al., 2021 18 |
Constipation (14, 37.8%) |
Neutropenia (10; 31.3%) |
Pneumonia (2; 6.25%) |
Chicken pox (01; 3.13%) |
Acute febrile Illness (03; 9.4%) |
Dengue infection (2; 6.25%) |
Dizziness* (05; 15.63%) |
AKI* (01; 3.13%) |
Sedation (03; 9.4%) |
-------- |
-------- |
|
Yang K et al., 2020 22 |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
|
Nag A et al., 2020 20 |
Constipation (10, 47%) |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
|
Yang K et al., 2020 21 |
Peripheral neurotoxicity* (01; 4.4%) |
Rash (02; 8.6%) |
Menstrual disorder (02; 8.6%) |
GIT disorders (05; 21.7%) |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
|
Yassin AK 2019 19 |
Constipation (10; 71.4%) |
EHAM (01; 7.14%)* |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
-------- |
EHAM: Extramedullary Hemopoietic Abdominal Masses, AKI: Acute Kidney Injury, CVT: Central Venous Thrombosis, DVT: Deep Vein Thrombosis, GIT: Gastrointestinal Tract, (*): Patients withdrew thalidomide due to adverse event (s)
DISCUSSION
Three studies involving a total of 780 patients met the eligibility criteria and were included in this review. All the studies were conducted prospectively. The findings demonstrated that thalidomide therapy led to transfusion independence in 69.6% (n=543) of patients. Adverse effects were reported in 41.2% (n=322) of patients, with most being mild to moderate, allowing patients to continue therapy despite these side effects. However, fourteen patients discontinued treatment due to severe adverse effects. The most commonly reported side effect was constipation (n=81), followed by dizziness (n=50). The mean duration of therapy was 13 months, and no mortality was reported during the treatment period. The studies showed significant improvements in hemoglobin levels, HbF%, serum ferritin, and spleen size from baseline to the end of follow-up. One study also assessed the quality of life.11
Patients with TDBTM require regular blood transfusions for survival, but chronic transfusions often result in iron overload complications. TDBTM patients absorb significantly more iron compared to healthy individuals. In TDBTM patients, iron levels can reach up to 250 mg/unit of blood, whereas healthy individuals absorb only about 0.5 g of iron annually.23 Excess iron is toxic to several organs, as it generates reactive oxygen species (ROS), which initiate auto-catalytic reactions with cellular components. These oxygen-derived free radicals damage lipids, proteins, and DNA within cells.24, 25 The organs most commonly affected by iron overload include the heart, pancreas, adrenal glands, reproductive organs, joints, and liver.26, 27
In low-income countries, iron chelation therapy is the most common treatment for TDBTM patients.28 Treatment strategies for beta-thalassemia major include regular blood transfusions and iron chelation therapy, and in some cases, HSCT.7 However, for patients who cannot afford these treatment options, thalidomide offers a cost-effective and safe alternative. Studies have shown that long-term use of thalidomide can significantly increase hemoglobin levels.29
This systematic review suggests that thalidomide therapy can improve the quality of life and reduce the number of transfusions required for TDBTM patients. The burden of regular blood transfusions and iron chelation therapy places considerable stress on both the families of these patients and the healthcare systems. For instance, the annual cost of blood transfusions for a 4-year-old thalassemia child ranges between US$ 1948 and US$ 2164.30 Additionally, the total annual cost for treating 130 beta-thalassemia major patients in Mumbai was estimated to be US$ 127,535.31 In Sri Lanka, the treatment of beta-thalassemia major imposes a significant financial strain on healthcare services and the families of affected children.32
For these reasons, thalidomide treatment can minimize both the medical complications and the financial burden on patients and their families. This systematic review recommends the use of thalidomide as an affordable and accessible treatment option for TDBTM patients.
CONCLUSION
Thalidomide is generally well tolerated in transfusion-dependent beta-thalassemia patients, with minimal harmful reactions and no major risks identified. It demonstrates promising potential in reducing the need for regular blood transfusions. However, this systematic review highlights the need for additional research, particularly rigorously designed clinical trials, to validate these findings, establish the long-term safety and efficacy of thalidomide, and optimize its use in TDBTM patients.
Limitations of the study
The study has several limitations that may impact the interpretation of the findings. Firstly, the review included only nine studies, which may not provide a comprehensive overview of thalidomide's efficacy and safety in TDBTM. Additionally, the included studies exhibited heterogeneity in design, patient populations, and outcome reporting, complicating comparisons and generalizability. Many studies had relatively short follow-up durations, limiting the assessment of long-term effects. There is also a potential risk of bias in study selection, as non-English studies have been excluded. The quality of the included studies varied, with some presenting moderate to low-quality evidence, which raises concerns about reliability. Furthermore, the absence of control groups in some studies and reliance on self-reported data may introduce bias in evaluating treatment effectiveness and adverse effects. Furthermore, one study by Ali Z et al., 16 lacked adequate justification for its sample size, potentially limiting the generalizability of the results. Collectively, these limitations highlight the need for further research to validate the findings and provide a more thorough understanding of thalidomide's role in TDBTM management.
REFERENCES
1. Aydinok Y. Thalassemia. Hematology 2012;17(sup1):s28-s31. https://doi.org/10.1179/102453312X13336169155295
2. Goh LPW, Chong ETJ, Lee P-C. Prevalence of Alpha(α)-Thalassemia in Southeast Asia (2010–2020): A Meta-Analysis Involving 83,674 Subjects. Int J Environ Res Public Health 2020;17(20):7354. https://doi.org/10.3390/ijerph17207354
3. Sayani FA, Kwiatkowski JL. Increasing prevalence of thalassemia in America: Implications for primary care. Ann Med 2015;47(7):592-604. https://doi.org/10.3109/07853890.2015.1091942
4. Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis 2010;5(1):11. https://doi.org/10.1186/1750-1172-5-11
5. Joly P, Lacan P, Garcia C, Couprie N, Francina A. Identification and molecular characterization of four new large deletions in the β-globin gene cluster. Blood Cells Mol Dis 2009;43(1):53-7. https://doi.org/10.1016/j.bcmd.2009.01.017
6. Li Y, Ren Q, Zhou Y, Li P, Lin W, Yin X. Thalidomide has a significant effect in patients with thalassemia intermedia. Hematology 2018;23(1):50-4. https://doi.org/10.1080/10245332.2017.1354427
7. Shah FT, Sayani F, Trompeter S, Drasar E, Piga A. Challenges of blood transfusions in β-thalassemia. Blood Rev 2019;37:100588. https://doi.org/10.1016/j.blre.2019.100588
8. Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil J-A, Hongeng S, et al. Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. New Eng J Med 2018;378(16):1479-93. https://doi.org/10.1056/NEJMoa1705342
9. Cappellini MD, Viprakasit V, Taher AT, Georgiev P, Kuo KHM, Coates T, et al. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent β-Thalassemia. New Eng J Med 2020;382(13):1219-31. https://doi.org/10.1056/NEJMoa1910182
10. Vichinsky E, Neumayr L, Trimble S, Giardina PJ, Cohen AR, Coates T, et al. Transfusion complications in thalassemia patients: a report from the Centers for Disease Control and Prevention (CME). Transfusion 2014;54(4):972-81. https://doi.org/10.1111/trf.12348
11. Chen J-M, Zhu W-J, Liu J, Wang G-Z, Chen X-Q, Tan Y, et al. Safety and efficacy of thalidomide in patients with transfusion-dependent β-thalassemia: a randomized clinical trial. Signal Transduct Target Ther 2021;6(1):405. https://doi.org/10.1038/s41392-021-00811-0
12. Zhu W, He Y, Huang M. Long-term follow-up of patients undergoing Thalidomide Therapy for transfusion-dependent β-Thalassaemia: A Single-Center Experience. Int J Gen Med 2024;17:1729-38. https://doi.org/10.2147/ijgm.s462991
13. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
14. NIH. (National Institute of Health). Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group 2021 [Accessed on: May 20, 2024]. Available from URL: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
15. Bhattacharjee U, Khadwal A, Shafiq N, Lad D, Sharma P, Das R, et al. A Phase 2 Randomized Controlled Trial of Single-Agent Hydroxyurea Versus Thalidomide Among Adult Transfusion Dependent β Thalassemia Patients. Indian J Hematol Blood Trans 2023;39(2):266-75. https://doi.org/10.1007/s12288-022-01620-3
16. Ali Z, Ismail M, Rehman IU, Rani GF, Ali M, Khan MTM. Long-term clinical efficacy and safety of thalidomide in patients with transfusion-dependent β-thalassemia: results from Thal-Thalido study. Sci Rep 2023;13(1):13592. https://doi.org/10.1038/s41598-023-40849-4
17. Li X, Hu S, Liu Y, Huang J, Hong W, Xu L, et al. Efficacy of Thalidomide Treatment in Children With Transfusion Dependent β-Thalassemia: A Retrospective Clinical Study. Front Pharmacol 2021;12. https://doi.org/10.3389/fphar.2021.722502
18. Chandra J, Parakh N, Sidharth, Singh N, Sharma S, Goel M, et al. Efficacy and Safety of Thalidomide in Patients With Transfusion-Dependent Thalassemia. Indian Pediatr 2021;58(7):611-6.
19. Yassin AK. Promising Response to Thalidomide in Symptomatic β-Thalassemia. Indian J Hematol Blood Trans 2020;36(2):337-41. https://doi.org/10.1007/s12288-019-01231-5
20. Nag A, Radhakrishnan VS, Kumar J, Bhave S, Mishra DK, Nair R, et al. Thalidomide in Patients with Transfusion-Dependent E-Beta Thalassemia Refractory to Hydroxyurea: A Single-Center Experience. Indian J Hematol Blood Trans 2020;36(2):399-402. https://doi.org/10.1007/s12288-020-01263-2
21. Yang K, Wu Y, Ma Y, Xiao J, Zhou Y, Yin X. The association of HBG2, BCL11A, and HBS1L-MYB polymorphisms to thalidomide response in Chinese β-thalassemia patients. Blood Cells Mol Dis 2020;84:102442. https://doi.org/10.1016/j.bcmd.2020.102442
>22. Yang K, Wu Y, Zhou Y, Long B, Lu Q, Zhou T, et al. Thalidomide for Patients with β-Thalassemia: A Multicenter Experience. Mediterr J Hematol Infect Dis 2020;12(1):e2020021. https://doi.org/10.4084/mjhid.2020.021
23. Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Diagnosis and treatment of cardiac iron overload in transfusion-dependent thalassemia patients. Exp Rev Hematol 2018;11(6):471-9. https://doi.org/10.1080/17474086.2018.1476134.
24. Faranoush P, Jahandideh A, Nekouian R, Mortazavi P. Evaluation of the in vitro and in vivo effect of liposomal doxorubicin along with oncolytic Newcastle disease virus on 4T1 cell line: Animal preclinical research. Vet Med Sci 2023;9(3):1426-37. https://doi.org/10.1002/vms3.1109
<25. Fleming RE, Ponka P. Iron Overload in Human Disease. New Eng J Med 2012;366(4):348-59. https://doi.org/10.1056/NEJMra1004967
26. Lekawanvijit S, Chattipakorn N. Iron overload thalassemic cardiomyopathy: Iron status assessment and mechanisms of mechanical and electrical disturbance due to iron toxicity. Canadian J Cardiol 2009;25(4):213-8. https://doi.org/10.1016/S0828-282X(09)70064-9
27. Wijarnpreecha K, Kumfu S, Chattipakorn SC, Chattipakorn N. Cardiomyopathy Associated with Iron Overload: How Does Iron Enter Myocytes and What are the Implications for Pharmacological Therapy? Hemoglobin 2015;39(1):9-17. https://doi.org/10.3109/03630269.2014.987869
28. Bellotti D, Remelli M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021;26(11):3255. https://doi.org/10.3390/molecules26113255.
29. Aguilar-Lopez LB, Delgado-Lamas JL, Rubio-Jurado B, Javier Perea F, Ibarra B. Thalidomide therapy in a patient with thalassemia major. Blood Cells Mol Dis 2008;41(1):136-7. https://doi.org/10.1016/j.bcmd.2008.03.001
30. Mallik S, Chatterjee C, Mandal PK, Sardar JC, Ghosh P, Manna N. Expenditure to treat thalassaemia: an experience at a tertiary care hospital in India. Iranian J Public Health. 2010;39(1):78-84.
31. Uchil A, Muranjan M, Gogtay NJ. Economic burden of beta-thalassaemia major receiving hypertransfusion therapy at a public hospital in Mumbai. Natl Med J India 2023;36(1):11-6. https://doi.org/10.25259/NMJI_580_20
32. Reed-Embleton H, Arambepola S, Dixon S, Maldonado BN, Premawardhena A, Arambepola M, et al. A cost-of-illness analysis of β-Thalassaemia major in children in Sri Lanka – experience from a tertiary level teaching hospital. BMC Pediatr 2020;20(1):257. https://doi.org/10.1186/s12887-020-02160-3
APPENDIX 1
Search terms: thalassaemia OR thalassemia OR Beta-thalassemia OR Beta-thalassaemia OR β-thalassaemia OR β-thalassemia OR b-thalassemia OR b-thalassaemia OR Alpha-thalassemia OR α-thalassemia OR Alpha-thalassaemia OR α-thalassaemia OR Sickle Cell Disease OR Sickle Cell Dis* OR SCD OR Anemia OR Anaemia OR Transfusion-dependent thalassemia OR Transfusion-dependent thalassaemia OR Blood transfu* OR hemoglobin synthesis OR haemoglobin OR hemoglobin OR fetal hemoglobin OR foetal hemoglobin OR foetal haemoglobin OR fetal haemoglobin OR HBF OR Fetal Hemoglobin Induc* OR Foetal Hemoglobin Induc* OR Foetal Haemoglobin Induc* OR Fetal Haemoglobin Induc* OR Hb F induc* AND Thalidomide OR thalidomid OR thalomid OR N-phthaloylglutamimide
AUTHORS' CONTRIBUTIONS Following authors have made substantial contributions to the manuscript as under:
TA: Conception and study design, acquisition, analysis and interpretation of data, drafting the manuscript, critical review, approval of the final version to be published ZS & WA: Acquisition, analysis and interpretation of data, critical review, approval of the final version to be published MN & NuA: Analysis and interpretation of data, drafting the manuscript, approval of the final version to be published
Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work.
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |