 https://doi.org/10.35845/kmuj.2024.23629 ORIGINAL
ARTICLE
https://doi.org/10.35845/kmuj.2024.23629 ORIGINAL
ARTICLE
Hepatoprotective
role of unacylated ghrelin in
different doses: an experimental study
Kumayl Abbas
Meghji  1,
Tariq Feroz Memon
1,
Tariq Feroz Memon  2, Muhammad
Shahab Hanif
2, Muhammad
Shahab Hanif  3,
Muhammad Saqib Baloch
3,
Muhammad Saqib Baloch  3, Ali
Abbas Thalho 4, Naila Noor 1
3, Ali
Abbas Thalho 4, Naila Noor 1
|
1: Department of
Physiology, Isra University, Hyderabad, Pakistan
2: Department of
Community Medicine and Public Health Sciences, Liaqat University of Medical
and Health Sciences, Jamshoro, Pakistan
3: Department of
Anatomy, Muhammad Medical College, Mirphurkhas, Pakistan
4: Department of
Pharmacology, Isra University, Hyderabad, Pakistan
5: Department of
Physiology, Muhammad Medical College, Mirphurkhas, Pakistan
Email
 : drtariqferoz@gmail.com : drtariqferoz@gmail.com
Contact #: +92-322-3490040
Date Submitted: March 03, 2024
Date Revised: August 26, 2024
Date Accepted: September 21,
2024
|
|
THIS ARTICLE MAY BE CITED AS: Meghji KA, Memon
TF, Hanif MS, Baloch MS, Thalho AB, Noor N. Hepatoprotective role of
unacylated ghrelin in different doses: an experimental study. Khyber Med Univ
J 2024;16(3):249-54. https://doi.org/10.35845/kmuj.2024.23629
|
ABSTRACT
OBJECTIVE: To investigate the
hepatoprotective effects of Unacylated Ghrelin (UAG) at varying doses in the
management of acute liver injury in Wistar albino rats.
METHODS: This quasi-experimental
study was conducted at Department of Physiology, Isra University, Hyderabad, Pakistan
from March to August 2023. Thirty Wistar albino rats (200-250 grams) were
randomly divided into five Groups (n=6). Group A served as the control, while
liver injury was induced in Groups B, C, D, and E via intraperitoneal injection
of 0.1% CCl₄. Groups C, D, and E were subsequently treated with low, medium,
and high doses of UAG, respectively. Serum alanine aminotransferase (ALT),
aspartate aminotransferase (AST), malondialdehyde, interleukin-6 (IL-6), tumor
necrosis factor-alpha (TNF-α), and superoxide dismutase (SOD) levels were
assessed, along with liver histopathology.
RESULTS: Pre-experimental body
weights (Mean±SD) for Groups A, B, C, D, and E were 227.33±7.75 g, 229.80±2.08
g, 228.70±5.34 g, 231.33±8.69 g, and 236.38±10.63 g, respectively. The liver
index was 4.36±0.28, 6.65±0.37, 5.80±0.17, 5.70±0.08, and 5.06±0.23,
respectively, across the Groups. A statistically significant (p<0.05)
decline was observed in group B compared to Group C, D and E. Moreover,
statistically significant (p<0.05) rise in ALT, AST, serum IL-6, TNFα, SOD,
and MDA levels in group B compared with the remaining Groups.
CONCLUSION: UAG effectively
protects the liver from CCl₄-induced injury in rats. Higher doses of UAG
reduced liver enzyme levels and improved oxidative stress and inflammation
markers, indicating its potential as a therapeutic agent for liver damage.
Further research is warranted to explore UAG's therapeutic use for liver
disorders.
KEYWORDS: Ghrelin (MeSH);
Oxidative Stress (MeSH); Liver Diseases (MeSH); Carbon Tetrachloride (MeSH)
Alanine Transaminase (MeSH); Aspartate Aminotransferases (MeSH); Malondialdehyde
(MeSH); Interleukin-6 (MeSH); Tumor Necrosis Factor-alpha (MeSH); Superoxide
Dismutase (MeSH); Histology (MeSH).
INTRODUCTION
The liver plays a
pivotal role in maintaining homeostasis and detoxifying harmful substances,
making it essential for survival. However, it is highly susceptible to damage
from various chemicals and toxins.1 Hepatic tissues can be harmed by
exogenous compounds like carbon tetrachloride (CCl₄), foreign chemicals, and
elevated cholesterol, leading to varying degrees of liver injury.2
Ghrelin, the only known natural ligand for the growth hormone secretagogue
receptor (GHSR), exists in both acylated and unacylated forms and is involved
in numerous biological processes.3 The receptor for ghrelin, GHSR1a,
is expressed in various organs, including the gastrointestinal tract (liver and
pancreas), cardiovascular system (heart), nervous system (hypothalamus,
pituitary, cerebral cortex), reproductive system (breast, testes, ovaries),
thyroid, and adrenal glands.4
Unacylated Ghrelin (UAG) is an
incarnation of the stomach ghrelin that accounts for 80-90% of the circulating
Ghrelin.5 UAG has demonstrated hepatoprotective
properties by preventing apoptosis and enhancing hepatocyte regeneration.6 With its anti-oxidative properties, it
minimizes the impact of oxidative stress that results from the production of
free radicals (reactive oxygen species) after acute liver injuries by
suppressing the silent information regulator 2 related enzyme 1 (sirtuin1,
SIRT1) signaling process. Moreover, the anti-inflammatory properties of UAG reduces
the inflammatory response linked to liver damage by lowering the production of
cytokines including TNF-α and IL-6.<7 Several studies have also alluded that
exogenous administration of UAG lowers the acylated Ghrelin/ Unacylated Ghrelin
circulatory ratio.7-9
While UAG’s anti-inflammatory, antioxidant, and
immune-modulatory effects have been documented, and its therapeutic potential
for treating acute liver injury is recognized, there remains a lack of
comprehensive data regarding its dose-dependent hepatoprotective effects. This
study was designed to explore the hepatoprotective
properties of exogenous UAG in different doses in in the management of acute
live injury in animal models.
METHODS
The quasi-experimental study was conducted by
the Department of Physiology, Isra University, Hyderabad, Pakistan from March
to August 2023. Thirty male Wistar albino rats between 200-250 g, were
purchased from the Animal Husbandry of Sindh Agricultural University, TandoJam,
Sindh, Pakistan.
The rats were housed under controlled
environmental conditions, maintaining an optimal temperature of 22±2°C and
humidity at 55±10%, with a regulated 12:12-hour light-dark cycle. After a
one-week acclimatization period, the experimental procedures commenced.
The study was approved by the Ethical Review
Committee of Isra University (ERB letter # IU/RR-10-IRC-23/N/2023/287) and
adhered to the international guidelines for the Care and Use of Laboratory
Animals.10
The
thirty rats were randomly divided into five Groups (n=6). Group A served as the
control, Group B was subjected to liver injury induction, and Groups C, D, and
E received varying doses of UAG. Specifically, Group C was administered 50
μg/kg of UAG, Group D received 100 μg/kg, and Group E was given 200 μg/kg, all
through intraperitoneal injections (NJPetide, Nanjing, China) for three
consecutive days. Three hours after the final UAG injection, all rats, except
those in the control group, were injected intraperitoneally with 0.1% CCl4
dissolved in corn oil to induce liver injury. The control group received only
corn oil at a volume of 0.1 mL per 10 g of body weight.
The
rats were weighed, and samples were collected 24 hours after liver injury
induction. All animals were sacrificed by cervical dislocation, and blood
samples were obtained via cardiac puncture. The collected serum was stored at
-20°C in sealed containers. Following the manufacturer's instructions, hepatic
markers, including serum alanine aminotransferase (ALT) and aspartate
aminotransferase (AST), as well as oxidative stress markers like
malondialdehyde (MDA) and superoxide dismutase (SOD), were analyzed using
commercial colorimetric kits. Moreover,
the inflammatory markers such as IL-6 and TNF-α levels were measured using
Solarbio ELISA kits (SEKR-0005-48T and SEKR-0009-48T, Beijing, China). After
blood sample collection, the liver was excised from each rat by dissecting the
abdominal cavity, weighed, and the liver index was calculated using the
following standard formula:11
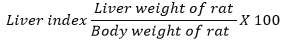
A
small section of the liver was excised and prepared in a 10% homogenate ice
saline solution, then submerged in 10% paraformaldehyde to create paraffin
sections. These sections were stored at -80°C. The paraffin-embedded sections
were sliced and stained with Hematoxylin and Eosin (H&E) for
histopathological examination. The analysis was performed using a light
microscope (Olympus CX31) at 100x magnification. Data were analyzed using SPSS
version 24. All quantitative variables were expressed as mean ± standard
deviation. One-way ANOVA followed by Post-hoc Tukey’s test was used to assess
significant differences between and within Groups. A p-value of <0.05 was
considered statistically significant.
RESULTS
The
pre-experimental body weight (Mean±SD) for Groups A, B, C, D, and E was
227.33±7.75 gm, 229.80±2.08 gm, 228.70±5.34 gm, 231.33±8.69 gm, and
236.38±10.63 gm, respectively. A significant difference in post-experimental
body weight was observed across all Groups. Group A showed an increase in weight
(249.0±23.78 gm), while Groups B (192.0±9.21 gm), C (219.10±2.14 gm), D
(226.63±7.56 gm), and E (233.66±11.57 gm) experienced weight reductions, as
illustrated in Figure 1. The difference between the Groups was statistically
significant, with a p-value of <0.05.
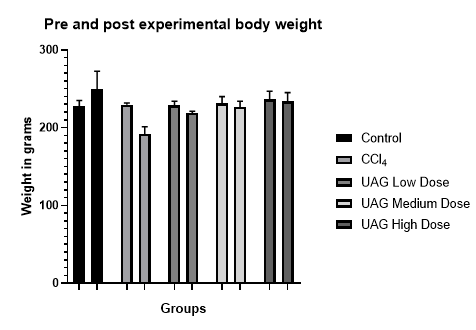
Figure 1: Distribution of pre and post-experimental body
weight among groups
The
distribution and post-hoc analysis of hepatic and inflammatory markers are
summarized in Table I. A statistically significant increase (p<0.05) in ALT,
AST, serum IL-6, and TNF-α levels was observed in group B. Although Groups C,
D, and E also showed an increase in these markers, the elevation was less
pronounced compared to group B, with group E demonstrating the most favorable
outcomes (p<0.05) (Table I).
Table I: Post-hoc
analysis of hepatic and inflammatory markers in all groups
|
Group
|
Group A
|
Group B
|
Group C
|
Group D
|
Group E
|
p-value
|
|
ALT
(U/L)
|
26.12±1.2bcd
|
168.3±9.3acde
|
147.0±8.8abde
|
76.16±3.5abce
|
34.5±3.0bcd
|
0.000*
|
|
AST
(U/L)
|
27.66±1.8 bcd
|
90.33±3.7 acde
|
77.83±2.1 abde
|
48.33±1.6 abce
|
28.33±1.9 bcd
|
0.000*
|
|
Serum IL-6 (pg/ml)
|
108.33±8.1 bcde
|
189.83±9.1 acde
|
172.16±7.0 abde
|
157.83±1.9 abce
|
142.33±5.0 abcd
|
0.000*
|
|
TNFα (pg/ml)
|
104.5±4.5 bcde
|
289.66±9.8 acde
|
205±7.7 abe
|
204.33±7.9 abe
|
128.66±6.8 abcd
|
0.000*
|
ALT:
Alanine Aminotransferase; AST: Aspartate Aminotransferase; IL-6: Serum Interleukin-6;
TNFα: Tumor Necrosis Factor Alpha *ANOVA (statistically significant); data
presented as mean± SD
Regarding
the liver index of all study animals, significant differences (p<0.05) were
observed among the Groups. Rats in Group B exhibited a markedly increased liver
index compared to all other Groups. Although a rise in liver index was noted in
the experimental Groups, it was less pronounced than in Group B. Among the
experimental Groups, Group E demonstrated the most favorable results (Table II)
Table II: Post-hoc
analysis of liver index of variations between all groups
|
Group
|
Group A
|
Group B
|
Group C
|
Group D
|
Group E
|
p-value
|
|
Liver Index
|
4.36±0.28bcde
|
6.65±0.37acde
|
5.80±0.17abde
|
5.70±0.08abce
|
5.06±0.23abcd
|
0.000*
|
*ANOVA
(statistically significant); data presented as mean± SD
Table
III presents the oxidative stress markers distribution in all study Groups. An
increase in MDA level and a decrease in levels of SOD was observed in Group B
compared with other Groups. Whereas, post-induction treatment with UAG in the higher
dose Group (E) showed a significant improvement in MDA and SOD levels in comparison
with both treatment Groups (C and D).
Table
III: Post-hoc analysis of oxidative stress markers distribution in all study
groups
|
Group
|
Group A
|
Group B
|
Group C
|
Group D
|
Group E
|
p-value
|
|
MDA
(nmol/mL)
|
1.38±0.1bcde
|
3.21±0.3acde
|
2.61±0.1abde
|
1.90±0.1abc
|
1.8±0.06ab
|
0.000*
|
|
SOD
(U/mL)
|
113.8±9.9bcd
|
45.6±3.7ade
|
55.5±3.4ade
|
80.0±4.7abce
|
110.5±8.8bcd
|
0.000*
|
MDA=Malondialdehyde;
SOD=superoxide dismutase; *ANOVA (statistically significant); data presented as
mean± SD
Figure 2 illustrates the
histopathological changes across the study Groups. Group A (control) displayed
normal hepatic architecture. In contrast, Group B (CCl4-induced
liver injury) showed significant pathological alterations, including fatty
degeneration, lymphocyte infiltration, and extensive necrosis of the liver
parenchyma. Groups C (UAG low dose) and D (UAG medium dose) also displayed
similar histopathological changes, though lymphocytic infiltration and necrosis
were less pronounced compared to Group B. Group E (UAG high dose) demonstrated
near-normal hepatic architecture with preserved liver parenchyma and minimal
lymphocytic infiltration.
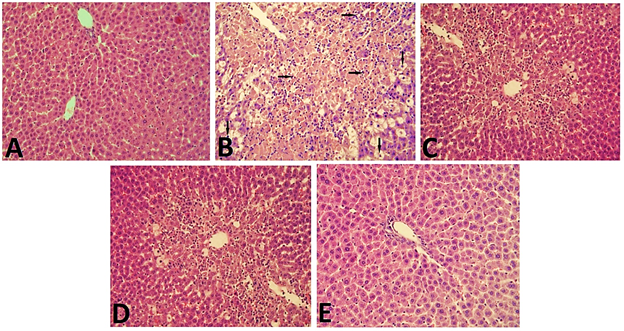
Figure
2: Evaluation of hepatic architecture in experimental animals (100x
magnification)
DISCUSSION
The
physiological effects of ghrelin's acylated form have been extensively studied
since its identification as a gut hormone. In contrast, its unacylated form,
previously considered inactive, has not received the same level of attention.
11, 12 However, recent
research suggests that UAG has significant physiological and pathological roles
that may complement or counteract the effects of acylated Ghrelin.13-15
This study aimed to evaluate the hepatoprotective properties of UAG in an acute
liver injury model using Wistar albino rats.
Carbon
tetrachloride (CCl4), a potent liver toxin, is commonly used in animal models
to induce liver damage. It is metabolized by the cytochrome P450 enzyme system,
producing reactive free radicals that cause oxidative stress and hepatocyte
injury.11, 16 In this study, Groups B, C, D, and E were subjected to
liver injury through intra-peritoneal injection of 0.1% CCl4, while Groups C,
D, and E received UAG in varying doses to assess its protective effects.
Significant
changes in body weights were observed pre- and post-experiment in all Groups,
with statistically significant differences between Groups B, C, D, and E compared
to the control group A. These findings align with previous studies by Gong Y, et
al.11 and Rossetti C, et al.17, which also reported
significant body weight variations in response to similar experimental
conditions.
In
the present study, a statistically significant increase in ALT and AST levels
was observed in Group B following intra-peritoneal CCl4 induction,
compared to the other study Groups. Conversely, the administration of UAG in
different doses resulted in a substantial and statistically significant
reduction in ALT and AST levels, with the most pronounced effects seen in Group
E (high-dose UAG). These results are consistent with findings from Gong et al.11 and Tuero et al.,18
who also reported the beneficial effects of UAG on elevated liver enzyme
levels.
After the induction of CCl4,
a statistically significant increase (p<0.05) in serum IL-6, TNFα, and MDA levels,
along with a decrease in SOD, was observed in Group B (induction group)
compared to the other Groups. Conversely, the administration of UAG
demonstrated notable hepatoprotective effects, attributed to its ability to
mitigate oxidative stress and inflammation induced by CCl4. This was
particularly evident in the high-dose UAG group (Group E), where a
statistically significant (p<0.05) improvement was observed.
CCl4 induces acute
liver damage through oxidative stress, which creates an imbalance between
pro-oxidants and antioxidants. Superoxide dismutase (SOD), an enzyme that
neutralizes free radicals, serves as a measure of hepatic antioxidant capacity.
Malondialdehyde (MDA), a byproduct of lipid peroxidation, indirectly indicates
the extent of liver damage caused by oxidative stress. Elevated MDA levels
correlate with increased liver cell damage and subsequent necrosis.
Additionally, inflammatory markers such as IL-6 and TNFα are elevated in
response to the liver injury.11
Numerous liver disorders are caused
by the strong inflammatory response and hepatocyte death that results from the
effects of TNFα.19 Moreover, serum
IL-6 contributes to the body's immunological response by encouraging
inflammation and exacerbating the oxidative stress response.20 Gong Y, et
al.,11, Raghay K, et al.,
21 and Bianchi E, et al. 22 demonstrated the similar effects of
serum IL-6, TNFα, SOD, and MDA levels and their effects on the liver and other
body cells. They further reported the protective effects of UAG against these
altered levels resulting from the CCl4 and other inducers.
Histological findings in this
study revealed that CCl4 induction led to significant damage to the
cell membrane, increasing permeability and causing hepatocyte injury in Group B
(induction group). The liver's architecture became disorganized, showing
necrosis, cellular breakdown, and inflammatory infiltration. In contrast,
treatment with UAG resulted in notable repair of liver damage, indicating a
potential dose-dependent hepatoprotective effect.
To the best of our knowledge,
this study is the first to explore the intervention effect of UAG on acute
liver injury in this context. Exogenous UAG appears to exert hepatoprotective
effects by reducing liver oxidative stress and modulating the inflammatory
response. The findings expand the understanding of UAG's pharmacological role
and may serve as a foundation for future research on UAG's potential in
managing liver disorders. However, this study represents only an initial
exploration of UAG's pharmacological activities. Further research is needed to
fully elucidate its therapeutic potential and underlying mechanisms.
CONCLUSION
This
study demonstrated that UAG exhibits significant hepatoprotective effects in
acute liver injury induced by CCl₄ in Wistar albino rats. UAG treatment,
particularly at higher doses, effectively mitigated liver damage as evidenced
by the significant reduction in liver enzyme levels (ALT and AST) and
improvement in oxidative and inflammatory markers. The study highlights the
potential of UAG as a therapeutic agent in managing acute liver injury,
suggesting its beneficial impact in reducing oxidative stress and inflammation
associated with liver damage. These findings support further investigation into
UAG's pharmacological properties and its potential applications in
liver-related disorders.
REFERENCES
1. Juanola A, Tiwari N, Solé C, Adebayo D, Wong F,
Ginès P. Organ dysfunction and failure in liver disease. Liver Int 2023 May 24. https://doi.org/10.1111/liv.15622
2.
Munir F, Khan MKA. Hepatotoxicity induced by carbon tetrachloride in
experimental model: hepatotoxicity induced by carbon tetrachloride. Pak BioMed
J 2023:10-5.
3. Müller TD, Nogueiras R, Andermann
ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab
2015;4(6):437-60. https://doi.org/10.1016/j.molmet.2015.03.005
4.
Ringuet MT, Furness JB, Furness SGB. G protein‐coupled receptor interactions
and modification of signalling involving the ghrelin receptor, GHSR1a. J
Neuroendocrinol 2022;34(9):e13077. https://doi.org/10.1111/jne.13077
5.
Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, et al.
Ghrelin reduces TNF-α–induced human hepatocyte apoptosis, autophagy, and
pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab
2019;104(1):21-37. https://doi.org/10.1210/jc.2018-01171
6. Quiñones M, Fernø J,
Al-Massadi O. Ghrelin and liver disease. Rev Endocr Metab Disord 2020;21:45-56. https://doi.org/10.1007/s11154-019-09528-6
7.
Lewiński A, Karbownik-Lewińska M, Wieczorek-Szukała K, Stasiak M, Stawerska R.
Contribution of ghrelin to the pathogenesis of growth hormone deficiency. Int J
Mol Sci 2021;22(16):9066. https://doi.org/10.3390/ijms22169066
8.
Hornsby AK, Buntwal L, Carisi MC, Santos VV, Johnston F, Roberts LD, et al.
Unacylated-ghrelin impairs hippocampal neurogenesis and memory in mice and is
altered in parkinson’s dementia in humans. Cell Rep Med 2020;1(7). https://doi.org/10.1016/j.xcrm.2020.100120
9.
Jabeen A, Ahsin S, Nasar Abbas ZS, Kiyani H. Ghrelin: A potent
nephro-protective agent against nicotine induced kidney damage in mice. Pak J
Med Health Sci 2023;17(01):171. https://doi.org/10.53350/pjmhs2023171171
10.
National Research Council (US) Committee for the Update of the Guide for the
care and use of laboratory animals. guide for the care and use of laboratory
animals. 8th edition. Washington (DC): National Academies Press
(US); 2011. [Accessed on: January 10, 2023]. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK54050.
https://doi.org/10.17226/12910
11. Gong Y, Qiu B,
Zheng H, Li X, Wang Y, Wu M, et al. Unacylated ghrelin attenuates acute liver
injury and hyperlipidemia via its anti-inflammatory and anti-oxidative
activities. Iran J Basic Med Sci 2024;27(1):49. https://doi.org/10.22038/IJBMS.2023.70831.15388
12.
Kiyani HP, Ahsin S, Imran M, Ashraf H. Effect of ghrelin in alleviating
nicotine induced oxidative stress in balb/C mice. Pak J Physiol 2022;18(3):3-6.
https://doi.org/10.69656/pjp.v18i3.1451
13.
Alharbi S. Exogenous administration of unacylated ghrelin attenuates hepatic
steatosis in high-fat diet-fed rats by modulating glucose homeostasis,
lipogenesis, oxidative stress, and endoplasmic reticulum stress. Biomed
Pharmacother 2022;151:113095. https://doi.org/10.1016/j.biopha.2022.113095
14. Au CC, Docanto MM,
Zahid H, Raffaelli F-M, Ferrero RL, Furness JB, et al. Des-acyl ghrelin
inhibits the capacity of macrophages to stimulate the expression of aromatase
in breast adipose stromal cells. The J Steroid Biochem Mol Biol 2017;170:49-53. https://doi.org/10.1016/j.jsbmb.2016.07.005
15.
Ugwu FN, Yu AP, Sin TK, Tam BT, Lai CW, Wong S, et al. Protective effect of
unacylated ghrelin on compression-induced skeletal muscle injury mediated by
SIRT1-signaling. Front Physiol 2017;8:962. https://doi.org/10.3389/fphys.2017.00962
16. Ramos-Tovar E, Muriel
P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis
in the liver. Antioxidants 2020;9(12):1279. https://doi.org/10.3390/antiox9121279
17.
Rossetti A, Togliatto G, Rolo AP, Teodoro JS, Granata R, Ghigo E, et al.
Unacylated ghrelin prevents mitochondrial dysfunction in a model of
ischemia/reperfusion liver injury. Cell Death Discov 2017;3(1):1-11. https://doi.org/10.1038/cddiscovery.2017.77
18.
Tuero C, Becerril S, Ezquerro S, Neira G, Frühbeck G, Rodríguez A. Molecular
and cellular mechanisms underlying the hepatoprotective role of ghrelin against
NAFLD progression. J Physiol Biochem 2023;79(4):833-49. https://doi.org/10.1007/s13105-022-00933-1
19.
Jing Z-T, Liu W, Xue C-R, Wu S-X, Chen W-N, Lin X-J, et al. AKT activator SC79
protects hepatocytes from TNF-α-mediated apoptosis and alleviates
d-Gal/LPS-induced liver injury. Am J Physiol-Gastrointest Liver Physiol
2019;316(3):G387-G96. https://doi.org/10.1152/ajpgi.00350.2018
20.
Guo Y, Gao S, Jiang Z, Huang J, He X, Jin R, et al. Calcium-sensing receptor
(CaSR) agonist R568 inhibits small intestinal motility of mice through neural
and non-neural mechanisms. Food Function 2021;12(23):11926-37. https://doi.org/10.1039/d1fo01988k
21.
Raghay K, Akki R, Bensaid D, Errami M. Ghrelin as an anti-inflammatory and
protective agent in ischemia/reperfusion injury. Peptides 2020;124:170226. https://doi.org/10.1016/j.peptides.2019.170226.
22.
Bianchi E, Boekelheide K, Sigman M, Hall SJ, Hwang K. Ghrelin modulates
testicular damage in a cryptorchid mouse model. PLoS One 2017;12(5):e0177995. https://doi.org/10.1371/journal.pone.0177995
AUTHORS' CONTRIBUTIONS
Following authors have made substantial contributions to the
manuscript as under:
KAM:
Conception and study design, acquisition of data, drafting the
manuscript, approval of the final version to be published
TFM:
Acquisition, analysis and interpretation of data, drafting the
manuscript, approval of the final version to be published
MSH
& MSB: Acquisition of data, drafting the manuscript, approval of the
final version to be published
AAT
& NN: Analysis and interpretation of data, critical review, approval
of the final version to be published
Authors agree to be accountable for
all aspects of the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated and
resolved.
|
|
CONFLICT
OF INTEREST
Authors declared
no conflict of interest, whether financial or otherwise, that could influence
the integrity, objectivity, or validity of their research work.
GRANT
SUPPORT AND FINANCIAL DISCLOSURE
Authors declared
no specific grant for this research from any funding agency in the public,
commercial or non-profit sectors
|
|
DATA
SHARING STATEMENT
The data that support the findings of this study are
available from the corresponding author upon reasonable request
|
|
 This is an Open Access article distributed under the terms of
the Creative Commons Attribution 4.0 International License. This is an Open Access article distributed under the terms of
the Creative Commons Attribution 4.0 International License.
|
![]() https://doi.org/10.35845/kmuj.2024.23629 ORIGINAL
ARTICLE
https://doi.org/10.35845/kmuj.2024.23629 ORIGINAL
ARTICLE![]() 1,
Tariq Feroz Memon
1,
Tariq Feroz Memon ![]() 2, Muhammad
Shahab Hanif
2, Muhammad
Shahab Hanif ![]() 3,
Muhammad Saqib Baloch
3,
Muhammad Saqib Baloch ![]() 3, Ali
Abbas Thalho 4, Naila Noor 1
3, Ali
Abbas Thalho 4, Naila Noor 1
