![]() https://doi.org/10.35845/kmuj.2024.23594
ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2024.23594
ORIGINAL ARTICLE
The role of SATB2, CDX2, CK7 and CK20 in differentiating primary versus metastatic ovarian carcinoma
Sana Tahir ![]() 1 , Lubna Avesi
1 , Lubna Avesi ![]() 1, Saba Hassan Shamim
1, Saba Hassan Shamim ![]() 1, Farheen Danish
1, Farheen Danish ![]() 1, Uzma Bukhari
1, Uzma Bukhari ![]() 1, Farah Muhammad Ali
1, Farah Muhammad Ali ![]() 1
1
|
1: Department of Histopathology, Dow University of Health Sciences (DUHS), Karachi, Pakistan
Email
Contact #: +92-313-8939149
Date Submitted: January 28, 2024 Date Revised: September 30, 2024 Date Accepted: September 30, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Tahir S, Avesi L, Shamim SH, Danish F, Bukhari U, Ali FM. The role of SATB2, CDX2, CK7 and CK20 in differentiating primary versus metastatic ovarian carcinoma. Khyber Med Univ J 2024;16(3):244-8. https://doi.org/10.35845/kmuj.2024.23594 |
ABSTRACT
OBJECTIVE: To find out the association of primary ovarian carcinomas (POC) and metastatic gastrointestinal carcinoma (MGC) by utilizing a panel of antibodies including Cytokeratin (CK) 7, CK20, Caudal-type Homeobox Transcription Factor-2 (CDX2), and SATB2 with their clinicopathological parameters.
METHODS: This prospective cross-sectional study was conducted from Mar, 2023 to Dec 2023 at Dow University of Health Sciences, Karachi, Pakistan. Biopsy tissue specimens from female patients over the 40 years, diagnosed with primary and metastatic ovarian carcinomas (MOC), were included. Immunohistochemistry, employing a three-tier scoring system, assessed CK7, CK20, CDX2, and SATB2 expressions. Statistical analysis was performed, utilized SPSS version 26.0 by employing Chi-Square test.
RESULTS: Over a 9-month period, 100 biopsied tissue samples were collected from patients aged 42-71 years (mean age 53.24 ± 8.03 years). Among these, 79 were POC (51% serous carcinoma, 9% mucinous carcinoma, 18% endometrioid carcinoma, and 1% clear cell carcinoma), and 21 were MGC. CK7 showed diffuse staining predominantly in POC and was absent in MGC (p < 0.001). CK20 exhibited significant focal and diffuse staining in MGC and was largely absent in POC (p = 0.005). CDX2 was significantly absent in POC and showed diffuse and focal staining in MGC (p < 0.001). SATB2 was absent in POC and displayed focal or diffuse staining in MGC (p < 0.001).
CONCLUSION: The inclusion of SATB2 in immunohistochemical panels, alongside CK7, CK20, and CDX2, improves the accuracy of diagnosing primary and metastatic ovarian carcinomas. This approach provides support in the distinction between ovarian and gastrointestinal tumors.
KEY WORDS: Ovarian Neoplasms (MeSH); Primary and metastatic ovarian carcinoma (Non-MeSH); CK7 (Non-MeSH); CK20 (MeSH); CK20 protein, human (MeSH); CDX2 (MeSH); SATB2 (MeSH); Immunohistochemistry (MeSH).
INTRODUCTION
Ovarian cancer (OC) presents a significant global health challenge, ranking seventh among malignant tumors in women and eighth in cancer-related mortality. Its high mortality rate makes it the third leading cause of cancer deaths among women, following cervical and uterine cancers.1 Among the various histological subtypes, epithelial ovarian cancer is the most prominent, consisting of four main categories: serous, endometrioid, clear cell, and mucinous tumors. Each subtype exhibits distinct biological characteristics and varying treatment responses. Additionally, there are rarer subtypes, including Brenner and seromucinous tumors.2
Ovarian cancer can be further categorized into two subtypes: Type I and Type II tumors. Type II tumors are generally more lethal, often linked to factors like continuous ovarian cycles, inflammation, and endometriosis. Type I tumors include low-grade serous, endometrioid, clear-cell, and mucinous carcinomas, with rarer forms such as seromucinous and Brenner tumors. These tumors typically present at an early stage, are predominantly low grade, and exhibit low proliferative activity, resulting in a more favorable prognosis. In contrast, Type II tumors are high-grade and usually diagnosed at an advanced stage. They are characterized by aggressive progression, heightened proliferative activity, significant chromosomal instability, and often involve p53 mutations.2,3
Despite advancements in understanding ovarian cancer subtypes, distinguishing primary ovarian mucinous neoplasms from metastatic tumors, especially those from the lower gastrointestinal tract, remains challenging. Immunohistochemical markers, including cytokeratin 7 (CK7), cytokeratin 20 (CK20), and Caudal-type Homeobox Transcription Factor 2 (CDX2), are valuable tools for this differentiation. CK7 is often positive in primary mucinous ovarian carcinomas, but it can also be found in colon and rectal carcinomas, complicating its diagnostic use. CK20 typically shows diffuse positivity in metastatic colorectal carcinomas, yet it may also appear focal or diffuse in primary ovarian mucinous neoplasms. CDX2, a nuclear transcription factor involved in intestinal differentiation, is expressed in both colon carcinoma and mucinous ovarian neoplasms, further obscuring the distinction between primary and metastatic tumors.4-6
This study addresses the clinical challenges in differentiating mucinous ovarian neoplasms, especially in distinguishing primary tumors from metastatic ones originating in the lower gastrointestinal tract, such as colorectal adenocarcinomas and appendiceal neoplasms. The challenge arises from overlapping immunohistochemical profiles that complicate accurate diagnosis. Accurate diagnosis is crucial, as it significantly impacts prognosis and treatment strategies. With the rise of targeted therapies, understanding the biological profiles of these tumors could open new therapeutic pathways for future clinical trials.
The primary aim of this study was to investigate the association between the immunohistochemical profiles of primary ovarian carcinoma (POC) and metastatic gastrointestinal carcinoma (MGC) using a panel of antibodies. This included traditional markers like CK7, CK20, and CDX2, as well as novel markers such as SATB2.
METHODS
This prospective cross-sectional study was conducted from March to December 2023, at the Histopathology Department of the Dow Diagnostic, Research and Reference Laboratory at Dow University of Health Sciences (DUHS) in Karachi, Pakistan. Convenience sampling was used, applying strict inclusion criteria that involved tissue specimens from female patients over 40 years old, including both primary and secondary ovarian carcinomas, provided they consented to participate. Exclusion criteria ruled out poorly fixed tissues, improperly sectioned or stained slides, requisition forms lacking adequate clinical details, benign ovarian lesions identified via light microscopy, patients under 40, and those who had received chemotherapy or radiation therapy. The study's dependent variables included patients diagnosed with ovarian and gastrointestinal malignancies, while independent variables comprised SATB2 expression, risk factors, socioeconomic status, ethnicity, and tumor grading and staging.
Tissue specimens suspected of ovarian malignancy were collected and processed according to internationally recognized grossing protocols in the histopathology department. Initial diagnoses were made using light microscopy on Hematoxylin and Eosin-stained slides, and only specimens confirmed as carcinomas were included in the study after rigorous review by consultant pathologists.
Tissue processing for Immunohistochemistry: Immunohistochemistry was conducted on formalin-fixed, paraffin-embedded (FFPE) tissue sections, which were deparaffinized, rehydrated, and treated for epitope retrieval and endogenous peroxidase inactivation. After primary antibody incubation using ready-to-use DAKO Mouse, secondary antibody incubation (DAKO, HRP [Code K4065]) followed for 30 minutes.
Results were interpreted by evaluating immunohistochemical staining of tumor cells: CK7 and CK20 positivity in epithelial cells, and nuclear staining for CDX2 and SATB2. Marker expression was classified using a three-tier system: "Absent" for no detectable staining, "Focal" for 1–50% of tumor cells, and "Diffuse" for over 50%, considering specific subcellular locations during evaluation. 7
Ethical approval: Ethical considerations were crucial in this study, with approval obtained from the Institutional Review Board (IRB) of DUHS, Karachi (REF #IRB-2905/DUHS/Approval/2023/110). Patient confidentiality was rigorously upheld, and no harm was inflicted on participants throughout the research.
Statistical analysis: Data were entered and analyzed using IBM SPSS version 26.0. Descriptive statistics and Chi-square tests assessed the association between histopathological patterns and clinical hysterectomy diagnoses, with statistical significance set at a p-value of <0.05.
RESULTS
Over a 9-month period, 100 samples were collected from patients aged 42 to 71 years (mean age 53.24 ± 8.03 years). Among these, 79 tumors (79%) were classified as primary ovarian cancers:
(serous carcinoma accounted for 51%, mucinous carcinoma represented 9%, endometrioid carcinoma (EC) comprised 18%, and clear cell carcinoma constituted for only 1%).The remaining 21 tumors were identified as metastatic gastrointestinal carcinomas.
Analysis of tumor size showed that most primary ovarian tumors, particularly serous carcinomas (SC), were larger than 10 cm, while mucinous carcinomas (MC) and endometrioid carcinomas (EC) varied in size. Unilateral presentation was common across all histological types, especially in SC, whereas bilateral cases were mainly associated with metastatic gastrointestinal tumors. Nodal involvement was limited, primarily occurring in metastatic gastrointestinal carcinomas (MGC) [Table I].
Table I: Characteristics of ovarian cancers
|
Characteristics |
SC |
MC |
EC |
CCC |
MGC |
Total |
|
|
Size |
<10cm |
21 |
3 |
3 |
0 |
14 |
41 |
|
>10cm |
30 |
6 |
15 |
1 |
7 |
59 |
|
|
Tumor Laterality |
Unilateral |
49 |
7 |
17 |
1 |
4 |
78 |
|
Bilateral |
2 |
2 |
1 |
0 |
17 |
22 |
|
|
Nodal Involvement |
Involved |
1 |
0 |
2 |
0 |
13 |
16 |
|
Not Involved |
50 |
9 |
16 |
1 |
8 |
84 |
|
SC: serous carcinomas; MC: mucinous carcinoma; EC: endometrioid carcinomas; CCC: clear cell carcinoma;
MGC: metastatic gastrointestinal carcinomas
Immunohistochemical Analysis: SC exhibited a distinct immunohistochemical profile, with predominantly focal and diffuse staining for CK7, absent CK20, and consistently absent CDX2 and SATB2. Mucinous carcinoma (MC) showed primarily focal CK7 staining, CK20 was mostly absent, while CDX2 and SATB2 presented with focal or diffuse staining. EC displayed varied staining patterns, and clear cell carcinoma (CCC) generally showed absent staining for SATB2 and CK20, though focal positivity for CK7 and CDX2 was noted (Figure 2). In metastatic gastrointestinal carcinoma (MGC), CK7, CK20, CDX2, and SATB2 predominantly exhibited diffuse staining (Figure 3). Statistically significant associations (p < 0.001) were found between the expression of these markers in POC and MGC (Table II).
Table II: Immunohistochemical results of ovarian cancers
|
Variables |
SC |
MC |
EC |
CCC |
MGC |
p-value |
|
|
CK7 |
Absent |
3 |
0 |
2 |
0 |
17 |
<0.001 |
|
Focal |
30 |
2 |
13 |
1 |
4 |
||
|
Diffuse |
18 |
7 |
3 |
0 |
0 |
||
|
CK 20 |
Absent |
36 |
6 |
13 |
0 |
4 |
0.005 |
|
Focal |
14 |
3 |
4 |
0 |
13 |
||
|
Diffuse |
1 |
0 |
1 |
0 |
4 |
||
|
CD X2 |
Absent |
43 |
8 |
11 |
0 |
4 |
<0.001 |
|
Focal |
8 |
1 |
7 |
1 |
10 |
||
|
Diffuse |
0 |
0 |
0 |
0 |
7 |
||
|
SAT B2 |
Absent |
47 |
7 |
15 |
0 |
2 |
<0.001 |
|
Focal |
4 |
2 |
3 |
1 |
9 |
||
|
Diffuse |
0 |
0 |
0 |
0 |
10 |
||
SC: serous carcinomas; MC: mucinous carcinoma; EC: endometrioid carcinomas; CCC: clear cell carcinoma;
MGC: metastatic gastrointestinal; CK7: cytokeratin 7, CK20: cytokeratin 20, CD X2: caudal-type homeobox transcription factor 2
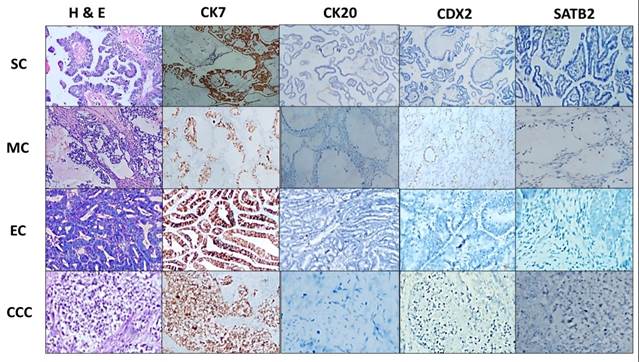
SATB2: Special AT-rich sequence-binding protein 2
Figure 1: Various Types of Primary Ovarian Cancers (H&E and Immunohistochemical Stains, 40x Magnification; Scale Bar: 500 μm)
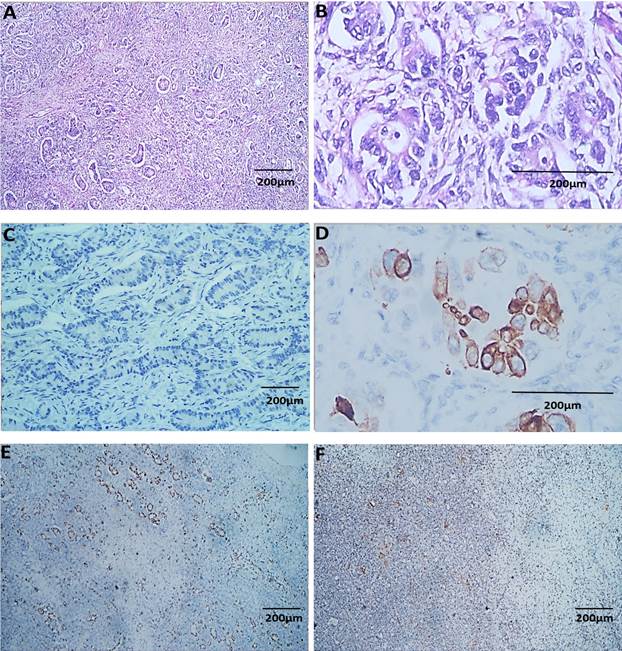
Figure 2: Metastatic Gastrointestinal Carcinoma. A & B: H & E, C: Diffuse Positive expression of CK7, D: Diffuse Positive expression of CK20, E: Diffuse Positive expression of CD X2, F: Diffuse Positive expression of SAT B2 (Magnification A, C, E & F: 40x; B & D: 400x) (Scale bar: 200 µm)
DISCUSSION
The present study highlights the key finding that metastatic ovarian tumors exhibit distinct immunohistochemical profiles compared to primary ovarian mucinous carcinomas. Specifically, metastatic carcinomas were positive for CK7, CK20, CDX2, and SATB2, while primary ovarian mucinous carcinomas showed positivity for CK7 and CK20, but negativity for CDX2 and SATB2. Notably, CDX2 was expressed in both primary ovarian mucinous carcinomas and metastatic gastrointestinal carcinomas, whereas SATB2 exhibited diffuse positivity exclusively in metastatic gastrointestinal tumors. This differentiation is critical, as metastatic tumors often share histological and immunohistochemical features with well-differentiated primary ovarian tumors, complicating the diagnostic process despite advanced tools such as macroscopic, microscopic, and histochemical evaluations. Therefore, an accurate diagnosis requires a comprehensive medical approach that takes into account these overlapping characteristics to distinguish between primary and metastatic ovarian tumors.8-10
Similar findings were observed in four distinct studies, which also investigated the immunohistochemical markers CK7, CK20, CDX2, and SATB2.11-15 These studies revealed a pattern consistent with the outcomes of our research. CK7 was predominantly expressed in POC, closely aligning with the trend identified in our study. In contrast, CK20, CDX2, and SATB2 were more commonly expressed in metastatic ovarian carcinomas (MOC). Moreover, these patterns were accompanied by statistically significant associations (p-value < 0.005), further corroborating our findings. 11,12
Our study investigated the immunohistochemical profiles of POC and MOC using the markers CK7, CK20, CDX2, and SATB2. We observed that a significant proportion of POC measured larger than 10 cm, whereas the majority of MOC were smaller, typically less than 10 cm in size. These findings are consistent with Strickland S, et al.,11 who reported similar results, and Mostafa et al.,12 whose study showed that a significant number of both primary and metastatic tumors exceeded 10 cm. Furthermore, in agreement with previous literature,11,12 our study found that most POC cases were unilateral, while MOC cases were predominantly bilateral. However, Montiel DP, et.,13 reported a contrasting outcome, indicating that both primary and metastatic tumors frequently exhibited bilateral involvement.
In the study by Meagher NS, et al.,7 a strong positive expression of CK7 was observed in 97% of primary ovarian carcinomas (POC), while its presence was notably lacking in metastatic ovarian carcinomas (MOC). Their findings align with our results, as CDX2 and SATB2 were predominantly expressed in MOC and were largely absent in POC. However, a discrepancy emerged regarding CK20; Meagher NS, et al. 7 reported its presence in both POC and MOC, whereas our study found a significant absence of CK20 in most POC cases compared to MOC.
Differentiating between POC and MOC is essential for patient prognosis and treatment planning. Early-stage POC has a high 5-year survival rate, while MOC often presents at a more advanced stage and is associated with a poorer prognosis.16,17 Our study highlights the critical role of immunohistochemical markers in accurately determining tumor origin, thereby facilitating appropriate therapeutic strategies and improving patient outcomes.
A primary limitation of our study was the relatively small sample size, which may affect the generalizability of our findings. Additionally, the study was conducted in a single center, potentially limiting the representation of the broader spectrum of ovarian tumor characteristics. This confinement could introduce selection bias, thereby affecting the external validity of the results. Another notable limitation was the exclusion of molecular or genetic analyses. Relying solely on immunohistochemical markers, without incorporating molecular profiling, may have restricted the depth and precision of our diagnostic approach.
To overcome these limitations, future research should focus on involving a larger and more diverse sample population to validate our findings. Multi-center studies across different geographic regions would allow for a broader representation of ovarian tumor characteristics and increase the generalizability of the results. Additionally, integrating molecular and genetic analyses alongside immunohistochemical markers could offer a more comprehensive understanding of tumor origins, potentially enhancing diagnostic accuracy and improving patient care strategies.
REFERENCES
1. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Women's Health 2019:287-99. https://doi.org/10.2147%2FIJWH.S197604
2. Arora T, Mullangi S, Lekkala MR. Ovarian Cancer. StatPearls Publishing, Treasure Island (FL). 2021.
3. De Leo A, Santini D, Ceccarelli C, Santandrea G, Palicelli A, Acquaviva G, et al. What is new on ovarian carcinoma: integrated morphologic and molecular analysis following the new 2020 World Health Organization classification of female genital tumors? Diagnostics 2021;11(4):697. https://doi.org/10.3390/diagnostics11040697
4. Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, et al. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol 2006;19(11):1421-8. https://doi.org/10.1038/modpathol.3800698
5. Shin JH, Bae JH, Lee A, Jung C-K, Yim HW, Park J-S, et al. CK7, CK20, CDX2 and MUC2 Immunohistochemical staining used to distinguish metastatic colorectal carcinoma involving ovary from primary ovarian mucinous adenocarcinoma. Jpn J Clin Oncol 2010;40(3):208-13. https://doi.org/10.1093/jjco/hyp150
6. Wang Y, Liu L, Yu Y. Mucins and mucinous ovarian carcinoma: development, differential diagnosis, and treatment. Heliyon 2023;9:e19221. https://doi.org/10.1016/j.heliyon.2023.e19221
7. Meagher NS, Wang L, Rambau PF, Intermaggio MP, Huntsman DG, Wilkens LR, et al. A combination of the immunohistochemical markers CK7 and SATB2 is highly sensitive and specific for distinguishing primary ovarian mucinous tumors from colorectal and appendiceal metastases. Mod Pathol 2019;32(12):1834-46. https://doi.org/10.1038/s41379-019-0302-0
8. Dundr P, Singh N, Nožičková B, Němejcová K, Bártů M, Stružinská I. Primary mucinous ovarian tumors vs. ovarian metastases from gastrointestinal tract, pancreas and biliary tree: a review of current problematics. Diag Pathol 2021;16(1):1-17. https://doi.org/10.1186/s13000-021-01079-2
9. Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynecol Obstet 2021;155(1):61-85. https://doi.org/10.1002/ijgo.13878
10. Kubeček O, Laco J, Špaček J, Petera J, Kopecký J, Kubečková A, et al. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin Exp Metast 2017;34(5):295-307. https://doi.org/10.1007/s10585-017-9856-8
11. Strickland S, Wasserman JK, Giassi A, Djordjevic B, Parra-Herran C. Immunohistochemistry in the diagnosis of mucinous neoplasms involving the ovary: the added value of SATB2 and biomarker discovery through protein expression database mining. Int J Gynecol Pathol 2016;35(3):191-208. https://doi.org/10.1097/PGP.0000000000000238
12. Mostafa NA, Fathi A. Validity of SATB2 in distinguishing metastatic mucinous carcinoma of colorectal origin from primary ovarian mucinous carcinoma. Egypt J Path 2020;18(1):4-9.
13. Montiel DP, Angulo KA, Cantú-de León D, Quevedo LB, Vilchis JC, Montalvo LH. The value of SATB2 in the differential diagnosis of intestinal-type mucinous tumors of the ovary: primary vs metastatic. Ann Diag Pathol 2015;19(4):249-52. https://doi.org/10.1016/j.anndiagpath.2015.05.004
14. Aldaoud N, Erashdi M, AlKhatib S, Abdo N, Al-Mohtaseb A, Graboski-Bauer A. The utility of PAX8 and SATB2 immunohistochemical stains in distinguishing ovarian mucinous neoplasms from colonic and appendiceal mucinous neoplasm. BMC Res Notes 2019;12(770):1-6. https://doi.org/10.1186%2Fs13104-019-4816-9
15. Li Z, Roth R, Rock JB, Lehman A, Marsh WL, Suarez A, et al. Dual immunostain with SATB2 and CK20 differentiates appendiceal mucinous neoplasms from ovarian mucinous neoplasms. Am J Clin Pathol 2017;147(5):484-91. https://doi.org/10.1093/ajcp/aqx023
16. Kurnit KC, Frumovitz M. Primary mucinous ovarian cancer: options for surgery and chemotherapy. Int J Gyne Can 2022;32(11). https://doi.org/10.1136/ijgc-2022-003806
17. Nasioudis D, Albright BB, Ko EM, Haggerty AF, Giuntoli RL, Burger RA, et al. Advanced stage primary mucinous ovarian carcinoma. Where do we stand? Arch Gynecol Obstet 2020;301:1047-54. https://doi.org/10.1007/s00404-020-05489-3
AUTHORS' CONTRIBUTIONS Following authors have made substantial contributions to the manuscript as under:
ST: Study design, acquisition of data, drafting the manuscript ,approval of the final version to be published LA: Acquisition of data, drafting the manuscript, approval of the final version to be published SHS: Analysis and interpretation of data, critical review, approval of the final version to be published FD, UB & FMA: Concept and study design, critical review, approval of the final version to be published
Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work.
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |