![]() https://doi.org/10.35845/kmuj.2024.23588 CASE
REPORT
https://doi.org/10.35845/kmuj.2024.23588 CASE
REPORT
Eosinophilic fasciitis as an immune-related adverse event of Pembrolizumab in Hodgkin’s lymphoma: a case report
Kamil
Khan1, Eithne Murphy1, Claire Louise Murphy![]() 1
1
|
1: Department of Rheumatology, Connolly hospital, Blanchardstown, Dublin D15 X40D, Ireland
Email
Contact #: +353-83-0628736
Date Submitted: January18, 2024 Date Revised: March14, 2024 Date Accepted: March 16, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Khan K, Murphy E, Murphy CL.Eosinophilic fasciitis as an immune-related adverse event of Pembrolizumab in Hodgkin’s lymphoma: a case report. Khyber Med Univ J 2024;16(2):171-4. https://doi.org/10.35845/kmuj.2024.234588 |
ABSTRACT:
BACKGROUND: Immune checkpoint inhibitors have transformed the treatment landscape for several types of cancer. These agents boost the patient's immune system by targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand. However, the enhanced immune activity can lead to a range of immune-related adverse events (irAEs), including rheumatological manifestations.
CASE PRESENTATION: We report a case of an 82-year-old male diagnosed with eosinophilic fasciitis. He had been receiving maintenance pembrolizumab at 200 mg every three weeks for the past three years for Hodgkin lymphoma. He presented with pain in his thumbs and wrists, accompanied by skin thickening and indurations of his forearms and lower limbs. Additionally, he reported myalgia, arthralgia, fatigue, and difficulty with manual dexterity. Blood tests revealed a normal full blood count, with no lymphopenia or eosinophilia. A connective tissue disease screen was negative, including tests for anti-centromere antibodies and anti-SCL 70. An MRI of the whole body showed fasciitis, predominantly in the thighs. A full-thickness skin biopsy revealed moderate infiltration by mononuclear lymphocytes and plasma cells. Pembrolizumab was discontinued, and the patient was started on 30 mg prednisolone with a gradual taper over three months. Additionally, methotrexate 20 mg once weekly was added to his regimen. Subsequently, the skin thickening and in duration in his forearms and lower limbs improved.
CONCLUSION: This case highlights eosinophilic fasciitis as a potential immune-related adverse event associated with pembrolizumab. Discontinuation of pembrolizumab and initiation of corticosteroids and methotrexate significantly improved the patient's symptoms and skin condition.
KEYWORDS: Eosinophilia (MeSH);Eosinophilic fasciitis (Non-MeSH), Immune checkpoint inhibitor (Non-MeSH);Immune-related-adverse events (Non-MeSH).
INTRODUCTION
Eosinophilic fasciitis (EF) is a rare disorder similar to scleroderma.1 Unlike systemic sclerosis (SSc), EF is distinguished by the absence of Raynaud’s phenomenon, organ involvement, and auto antibodies. Possible causes of EF include medications, idiopathic causes and haematological malignancies. EF typically affects the extremities while sparing the trunk and face, causing skin edema, indurations, and occasionally contractures, which can limit joint movement. EF may be associated with peripheral eosinophilia. There are no established treatment guidelines for this rare condition; however, patients with EF often respond well to steroids and oral immunosuppressants. This case is significant as it adds to the existing literature and may help inform future treatment protocols.
CASE PRESENTATION
We report the case of an 82-year-old man diagnosed with Hodgkin's lymphoma in 2016. Initially, he was treated with Chlorambucil, Vinblastine, Procarbazine, and Prednisolone. Following a relapse in 2019, he began maintenance therapy with Pembrolizumab at 3 mg/kg (200 mg) every three weeks. His disease remained stable, and a recent PET scan showed no recurrence of Hodgkin's lymphoma. After completing 56 cycles (3 years) of pembrolizumab, he developed pain in his thumbs and wrists.
Over six months, the pain in his thumbs and wrists gradually progressed to involve his forearms and legs, accompanied by skin thickening, myalgia, fatigue, and difficulty with manual activities and mobility. The patient denied experiencing Raynaud’s phenomenon, dysphagia, gastroesophageal reflux, dyspnea, or other connective tissue disease symptoms.
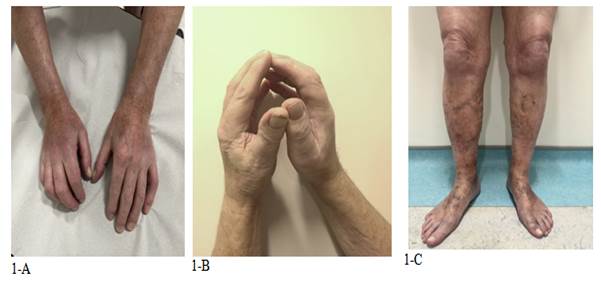
He was admitted to the Hematology department due to the progression of his musculoskeletal symptoms and was evaluated by both the rheumatology and dermatology teams. Clinical examination revealed thickened, indurated, and tender skin on his arms, forearms, and lower limbs, with no involvement of the torso or face and no signs of telangiectasia and prayer sign was positive (Figure 1A-C). Muscle power was 5/5 in all limbs.
Blood tests showed normal full blood count with normal eosinophil and lymphocyte counts. Renal function, ESR, glucose, HbA1C, and CK levels were within normal limits. CRP was high at 51. Immunoglobulins and serum protein electrophoresis (SPEP) were satisfactory. Connective tissue disease screen was negative for anti-centromere antibodies and anti-SCL 70.
Whole-body MRI revealed fasciitis primarily affecting the thighs, with STIR hyperintensity surrounding the posterior, medial, and anterior compartment muscles (Figure 2). A full-thickness skin biopsy showed fibro-adipose connective tissue with prominent blood vessels and moderate mononuclear infiltrates, including plasma cells.
The Rheumatology and Dermatology teams attributed the patient's eosinophilic fasciitis to Pembrolizumab, leading to its discontinuation. Detailed differential diagnosis ruled out other potential causes presenting similarly. Treatment began with a tapering regimen of 30 mg prednisolone, supplemented by Methotrexate 20 mg weekly with folic acid. After 2 months, pain and skin in duration showed improvement but persisted. The Hematology team opted for monitoring every 3 months for Hodgkin lymphoma recurrence, based on clinical and lab findings, including recent PET scan results. No additional immunosuppressive therapy was recommended. The patient continues to be regularly monitored.
|
Figure 1 (A, B, C:) Indurated and tender skin noted on the arms, forearms, and lower limbs without involvement of the torso or face. Positive prayer sign observed |
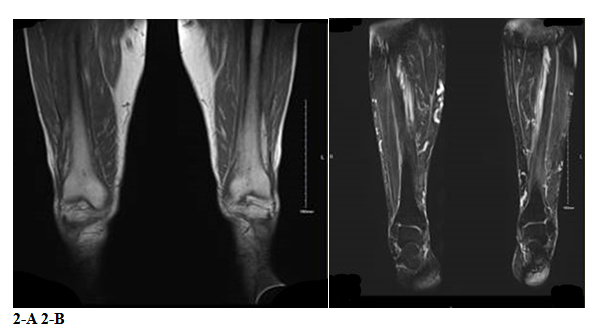
Figure 2: T2-weighted short-tau inversion recovery (STIR) imaging (2A) and T1 imaging (2B) of posterior, medial and anterior compartments muscles
Differential diagnosis: The mainrheumatological differential diagnosis of eosinophilic fasciitis is diffuse systemic sclerosis (SSc). However, Raynaud’ sphenomenon, internal organ involvement and auto antibodies were negative in this patient. Sclerodemadiabeticorum which can occur with long-standing diabetes mellitusmay have a similar presentation. However, our patient did not have diabetes mellitus. Nephrogenic systemic fibrosis can occur in patients with end-stagechronic kidney diseasewhoreceive IV gadolinium contrast. However, renal function was normal and there was no history of gadolinium contrast received. Scleroderma cases may be associated with multiple myeloma, however in our patient, immunoglobulins and SPEP was satisfactory.
DISCUSSION
Immune checkpoint inhibitors (ICI) are promising therapy for several cancers. The first ICI approved by FDA in 2011 is ipilimumab for melanoma. Later on, several ICI have been approved by FDA for a variety of malignancies, including non-small cell lung cancer, renal cell carcinoma, and Hodgkin’s lymphoma. ICI enhance endogenous T-cell-mediated patients’ immune response to cancer cells working by inhibiting the programmed cell death-1, PD-1 ligand and cytotoxic T-lymphocytes associated protein-4 on T cells and antigen-presenting cells.2 The path physiology of irAEs is still unclear. The development of immune-mediated effect is attributed to various hypothesized mechanisms, such as auto antibodies, T-cell infiltration, interleukins and other inflammatory cytokines.3
Over activation of the immune system may lead to immune-related adverse events in all organs, including the joints. It causes a variety of rheumatological manifestations of adverse events, including arthralgia, myalgia, inflammatory arthropathy, mystic, vasculitis, scleroderma, eosinophilic fasciitis and lupus nephritis.4Eosinophilic fasciitis isoften idiopathic or could be due to other causes such as medications (e.g. ICI including pembrolizumab),strenuous exercise, infection, haematological disorders and autoimmune diseases.5,6
Diagnosis of eosinophilic fasciitis is based on clinical findings as well as MRI and full thickness biopsy.7 Lab findings such as peripheral eosinophilia, and hypergammaglobulinemia are helpful but are not always necessary for diagnosis as per classification criteria. Eosinophilic fasciitis, in some cases resolved spontaneously.8EF responds well to steroids, commencing from 40-60 mg daily and taper down according to response to treatment. In some cases, IV steroids are given initially, followed by oral prednisolone.9Methotrexate is a good option as a steroid sparing agent, and is used in most cases. Other immune suppressants include my cophenolate mofetil, azathioprine, cyclophosphamide and rituximab. IL-5 is the main cytokine for eosinophil growth, differentiation, recruitment and activation.10 In refractory cases of EF associated with eosinophilia, IL-5 inhibition is effective therapy.
CONCLUSION
Eosinophilic fasciitis, a rare disease, is typically responsive to steroids and disease-modifying treatments, as highlighted by this case where it presented as an immune-related adverse event associated with pembrolizumab. Discontinuation of pembrolizumab and initiation of corticosteroids and methotrexate significantly improved the patient's symptoms and skin condition. A thorough patient history, including medication use, is crucial for identifying underlying causes early, which is essential in reducing morbidity associated with this condition.
REFERENCES
1. Pinal-Fernandez I, Selva-O' Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev 2014;13(4-5):379-82. https://doi.org/10.1016/j.autrev.2014.01.019.
2. Ihn H. Eosinophilic fasciitis: From pathophysiology to treatment. AllergolInt 2019;68(4):437-9. https://doi.org/10.1016/j.alit.2019.03.001
3. Dang QM, Watanabe R, Shiomi M, Fukumoto K, Nobashi TW, Okano T, et al. rheumatic immune-related adverse events due to immune checkpoint inhibitors—a 2023 update. Int J MolSci 2023;24(6):5643. https://doi.org/10.3390/ijms24065643
4. Cappelli LC, Gutierrez AK, Bingham CO, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017;69(11):1751-63. https://doi.org/10.1002/acr.23177
5. Amrane K, Le Meur C, Thuillier P, Alemany P, Niel C, Renault D, et al. Case report: Eosinophilic fasciitis induced by pembrolizumab with high FDG uptake on 18F-FDG-PET/CT. Front Med 2022;20(9)1078560. https://doi.org/10.3389/fmed.2022.1078560
6. Chan KK, Magro C, Shoushtari A, Rudin C, Rotemberg V, Rossi A, et al. Eosinophilic fasciitis following checkpoint inhibitor therapy: four cases and a review of literature. Oncologist 2020;25(2):140-9. https://doi.org/10.1634/theoncologist.2019-0508
7. Parker MJS, Roberts ME, Lorigan PC, Du Plessis DG, Chinoy H. Autoimmune fasciitis triggered by the anti-programmed cell death-1 monoclonal antibody nivolumab. BMJ Case Rep 2018; 2018: bcr2017223249. https://doi.org/10.1136/bcr-2017-223249
8. Mazori DR, Femia AN, Vleugels RA. eosinophilic fasciitis: an updated review on diagnosis and treatment. currrheumatol Rep 2017;19(12):74. https://doi.org/10.1007/s11926-017-0700-6
9. Bourcier L, St-Hilaire È, LeBlanc M, Picard L. Complete reversibility of pembrolizumab-induced eosinophilic fasciitis without corticosteroids: A case report. SAGE Open Med Case Rep 2021;9:2050313X211025111. https://doi.org/10.1177/2050313x211025111
10. Samborski W, Eaba R. AB0217 Intravenous pulse corticosteroid therapy in patient with eosinophilic fasciitis. Ann Rheum Dis 2001;60(Suppl 1):A290.2. https://doi.org/10.1136/annrheumdis-2001.738
Following authors have made substantial contributions to the manuscript as under:
KK: Identification of the case, drafting the manuscript, approval of the final version to be published
EM & CLM: Diagnosis and management of the case,critical review,approval of the final version to be published
Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work.
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |