![]() https://doi.org/10.35845/kmuj.2024.23558 ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2024.23558 ORIGINAL ARTICLE
Effects of noise stress on thyroid gland histomorphology in adult rats
Saba Saleem Safdar![]() 1,2, Khadija Qamar
1,2, Khadija Qamar![]() 1, Mehwish Abaid
1, Mehwish Abaid![]() 1 , Muhammad Fahad Atta
1 , Muhammad Fahad Atta![]() 1,3, Muhammad Sabir
1,3, Muhammad Sabir![]() 1,2, Muhammad Rizwan
Bashir Kiani
1,2, Muhammad Rizwan
Bashir Kiani![]() 1
1
|
1: Department of Anatomy, Army Medical College, National University of Medical Sciences (NUMS), Rawalpindi., Pakistan 2: Department of Anatomy, Sialkot Medical College, Sialkot, Pakistan 3: Department of Anatomy, Quaid-e-Azam Medical College, Bahawalpur, Pakistan
Email
Contact #: +92-323-2571749
Date Submitted: December19, 2023 Date Revised: March15, 2024 Date Accepted: March 29, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Safdar SS, Qamar K, Abaid M, Atta MF, Sabir M, Kiani MRB. Effects of noise stress on thyroid gland histomorphology in adult rats. Khyber Med Univ J 2024;16(2):165-70. https://doi.org/10.35845/kmuj.2024.23558 |
ABSTRACT:
OBJECTIVE: To analyze the effects of variable durations of loud noise stress on the histomorphology of the thyroid gland in adult rats.
METHODS: This laboratory-based experimental study was conducted at the Anatomy Department, Army Medical College/National University of Medical Sciences, Rawalpindi, Pakistan, from January to December 2020. Thirty Sprague Dawley rats were divided into three groups: Control group A (no noise exposure), experimental group B (100 dB noise for 4 hours daily), and experimental group C (100 dB noise for 6 hours daily) for four months. At the end of the experiment, the rats were weighed, euthanized, and their thyroid glands removed. The glands were analyzed microscopically after Haematoxylin and Eosin staining, measuring the diameter and epithelial height of thyroid follicles.
RESULTS: All rats remained healthy with no statistically significant differences in weight gain between the groups. The follicular epithelial height significantly increased in groups B (9.86±0.38 µm) and C (10.37±0.39 µm) compared to the control
group A, (p-values <0.001 and 0.001, respectively). The follicular diameter significantly decreased in groups B (86.61±7.68 µm) and C (95.47±5.24 µm) compared to group A, (p-values <0.001 and 0.003, respectively. Microscopic examination revealed that experimental groups exhibited disrupted thyroid follicles and increased inflammatory infiltrates compared to controls.
CONCLUSION: Exposure to variable durations of loud noise stress in adult rats leads to histomorphological changes in the thyroid gland, including increased follicular epithelial height and reduced follicular diameter. These findings are suggestive of potential hyperactivity of thyroid follicles in response to noise stress exposure.
KEYWORDS: Thyroid Gland (MeSH);Diameter (Non-MeSH); Epithelium (MeSH);Thyroid Follicle (Non-MeSH); Noise (MeSH); Stress (Non-MeSH);Stress, Physiological (MeSH); Thyroid Nodule (MeSH).
INTRODUCTION
Dr. Hans Selye first characterized stress as a syndrome caused by either a physical, mental, and/or psychological reaction of the body to any internal or external innocuous stimulus that needs to be adjusted accordingly.1 The body's homeostatic response toward stress varies depending on the magnitude, duration, and type of stressor. Noise is one of the major environmental stressors, defined as unwanted/undesirable sound which lacks a discernible pattern and might be perceived as unpleasant/irritating.2 Continuous noise exposure can exert prolonged effects in terms of impaired health, toxicity to environment, and economical losses.2,3 To prevent hearing loss, maximum 85 decibels noise exposure for average of 8 hours/day is the recommended safety limit.4
Long-term exposure to noise stress can lead to numerous health issues, including hearing impairment, sleep disorders, impaired attention, mental deficits, endocrine disruption, cardiovascular ailments, and metabolic derangements.5-7 Chronic exposure to loud noise drastically affects the quality of life by both occupational and non-occupational exposure such as vehicles, household appliances and leisure noise.3 According to the World Health Organization, noise-induced morbidities in European adults, measured as Disability-Adjusted Life Years (DALYs), were estimated to be 61,000 for cardiovascular diseases, 654,000 for mental annoyance, 903,000 for sleep disturbances, and 22,000 for tinnitus.7
To survive, the body adjusts its neurohormonal levels in response to stress by stimulating the sympathetic-adrenal-medullary system and the hypothalamic-pituitary-adrenal axis.8 This response of the body helps maintain homeostasis unless homeostatic limits are crossed, and stress response becomes detrimental to human wellbeing. The thyroid gland is closely regulated by the hypothalamic-pituitary axis.9 It can be speculated that thyroid gland can be directly or indirectly affected by stress exposure. Moreover, noise pollution reportedly affects all vital body functions.7 Thyroid hormones in turn are have potential contribution towards regulation of all these functions. Conditions like depression, fatigue, memory loss, and weight gain linked to noise stress also relate to thyroid dysfunction.10 Hence, adverse effects of environmental noise stress on structural and functional integrity of human thyroid gland is an important public health consideration.
Evidence on the effect of stress, particularly noise stress, on the thyroid gland's functioning and histomorphology in rodents and humans remains inconclusive due to the due to the limited data available.11-13 This is primarily because most studies have focused on the effects of stress in general, rather than specifically investigating noise stress. A cross sectional study, reported that occupational exposure to noise stress was associated with thyroid functional deregulation among 11.65% participants compared to 2.85% in the control group.14
Amidst rising global noise stress due to globalization and technological advancements, it is crucial to establish evidence on environmental noise's impact on thyroid gland structure and function. Given the concerning epidemiological data linking noise exposure to various health risks, there is an urgent necessity to establish substantial evidence regarding the influence of environmental noise stress on the structure and functions of the thyroid gland. The gap in literature exists as the effect of noise stress needs to be studied further on its effect on histomorphology of thyroid gland, which is the main gland that controls the metabolism of the entire body. The current study was planned to provide substantial evidence on this important public health issue by analyzing the effects of varying durations of loud noise stress on the histomorphology of the thyroid gland in adult rats, with implications for understanding similar impacts in humans.
METHODS
This experimental study was conducted at the Department of Anatomy, Army Medical College, Rawalpindi / National University of Medical Sciences (NUMS), in collaboration with the National Institute of Health (NIH), Islamabad, Pakistan. The research was conducted over a 12-month period, from January 2020 to December 2020, following ethical approval from the institutional review board (ERC/ID/08).Thirty Sprague Dawley rats were used which included equal number of male and female rats. Rats with any gross injuries or abnormalities were remove. Female pregnant rats were also excluded from the study. Healthy rats weighing 250±50 grams on average were included. Males and females were kept in separate cages to avoid mating.
The rats were split into three groups having ten rats each: group A served as the control group and did not experience any sound stress; group B and group C served as the experimental groups and were subjected to daily exposure to 100 dB noise for 4 and 6 hours, respectively. All rats had unlimited access to rat chow and water. Locally obtained pure tone noise generator was used to deliver noise stress, and a decibel meter (Radio Shack analogue model 33-4050) was used to check sound levels. Control group A was placed in a room which was far from the experimental groups room so that it should not be exposed to noise. The animal house is quite large and there are many enclosures and rooms for different animals. Experimental group B rats after being exposed to noise for 4 hours was taken to the same room as that of experimental group A so that the exposure to noise for experimental group C should be continued for the next 2 hours.
The rats' body weight was measured at the beginning and end of the experiment using a digital analytical balance, sensitive up to one-tenth of a gram increment. The reason for weight measurement is to check the overall effect of noise on the body’s metabolism as thyroid gland is mainly involved in metabolic processes of the body. At the conclusion of the study, the rats were euthanized using inhalant chloroform anesthetic overdose.15 The thyroid gland was carefully dissected out and washed with normal saline to remove excess blood. The biochemical tests were not performed because the gap in literature required histopathology of the thyroid gland to be studied further and the hormone levels were already extensively studied.
Olympus® Microscope Bx43 mounted with Olympus® Stylus 1010 Digital Camera (10 megapixels), was used for microscopic observation and photography of slides. All the images were analyzed using the software Civil AutoCAD version 2013.
At the end of the study, on microscopic observation, the diameter of thyroid follicles and follicular epithelial height were recorded from five thyroid follicles that were randomly selected from the prepared section of the thyroid gland of each animal, and their average was taken.16For thyroid follicle diameter, the average of maximum transverse diameter and its perpendicular was taken. The mean epithelial height of each thyroid follicle was calculated as an average of four equidistant measurements taken from basement membrane to follicular lumen. There was no grading of mild, moderate and severe for follicular diameter and height as these are quantitative parameters and not qualitative. The reference range was the mean value of control group A to which the values of experimental groups B and C were compared. It was done because group A rats were normal subjects living in completely normal conditions and hence, the values of all their parameters were normal and were considered as a reference.
RESULTS
All the rats in the study stayed healthy with a 100% survival rate. A one-way ANOVA was conducted to analyze the effects of noise stress exposure on the body weight gain of adult rats. The mean weight of rats did not differ statistically significantly between the groups at the beginning of the study (p = 0.8) and at the end of the study (p = 0.84). Rats in control group A and experimental groups B and C gained an average weight of 39 ± 27.26gm, 43 ± 23.11gm, and 43 ± 23.07gm, respectively, over 04 months.
Table I: Intergroup comparison of initial body weight of Adult Rats
|
Pair wise Groups |
Mean Difference |
p-value |
|
|
X |
Y |
x-y |
|
|
A |
B |
1 |
1.00 |
|
A |
C |
21 |
0.82 |
|
B |
C |
20 |
0.84 |
*p-value = significant at p ≤ 0.05 for post hoc Tukey Test (following significant overall F - test of One-way ANOVA)
Table II: Intergroup comparison of final body weight of adult rats
|
Pair wise Groups |
Mean Difference |
p-value |
|
|
X |
Y |
x-y |
|
|
A |
B |
-3.00 |
1.00 |
|
A |
C |
16.10 |
0.89 |
|
B |
C |
19.10 |
0.85 |
*p-value = significant at p ≤ 0.05 for post hoc Turkey Test (following significant overall F - test of One-way ANOVA)
Table III: Intergroup comparison of total body weight gain in Adult Rats
|
Pair wise Groups |
Mean Difference |
p-value |
|
|
X |
Y |
x-y |
|
|
A |
B |
-4.00 |
0.93 |
|
A |
C |
-4.90 |
0.90 |
|
B |
C |
-0.90 |
1.00 |
*p-value = significant at p ≤ 0.05 for post hoc Turkey Test (following significant overall F - test of One-way ANOVA)
The gross appearance and color of the thyroid gland among all groups was consistently normal. The location of the thyroid gland was found anatomically normal among all groups. One-way ANOVA was conducted to analyze the effect of noise stress exposure on the microscopic appearance of adult rats' thyroid glands. Table IV is showing that the mean follicular epithelial height of rats differed statistically significantly between the groups, p < 0.001. The experimental Group C rats had a higher mean follicular epithelial height as compared to the controlGroup A, a statistically significant difference of 3.74 µm, p < 0.001. Also, the experimental rats in experimental group B, had higher mean follicular epithelial height as compared to the control group A, a statistically significant difference of 3.24 µm, p< 0.001.
Table IV: Intergroup comparison of follicular epithelial height (µm) of rats
|
Pair wise Groups |
Mean Difference |
p-value |
|
|
X |
y |
x-y |
|
|
A |
B |
-3.24 |
<0.001* |
|
A |
C |
-3.74 |
0.001* |
|
B |
C |
-0.51 |
0.114 |
*p-value = significant at p ≤ 0.05 for post hoc Turkey Test (following significant overall F - test of One-way ANOVA)
Levine’s test for equality of variances determined that the assumption of homogeneity of variances was broken for thyroid follicular diameter (p = 0.026). Therefore, One-Way Welch's ANOVA with post hoc Games-Howell test was used to examine the impact of noise stress exposure on the microscopic structure of adult rats' thyroid glands in place of one-way ANOVA. Table V demonstrates that there was a statistically significant difference in the mean thyroid follicular diameter of the rats between the groups, p=0.001. The experimental group C rats had less mean thyroid follicular diameter as compared to the control group A, a statistically significant difference of 23.03 µm, p = 0.003. Games-Howell post hoc analysis revealed that experimental group C rats had a higher mean thyroid follicular diameter as compared to the experimental group B, a statistically significant difference of 8.85 µm, p = 0.021.
Table V: Intergroup comparison of thyroid follicular diameter (µm) in rats
|
Pair wise Groups |
Mean Difference |
p-value |
|
|
x |
Y |
x-y |
|
|
A |
B |
31.89 |
<0.001* |
|
A |
C |
23.03 |
0.003* |
|
B |
C |
-8.86 |
0.02* |
Microscopic examination revealed that the experimental group rats exhibited a higher proportion of disrupted thyroid follicles and interstitial than the control group rats. Thyroid follicles varied greatly in size and shape, but mostly the thyroid follicles among experimental groups were smaller than the control group, although majorly rounded in shape. A few large-sized follicles were also observed in the peripheral field. Follicles were loosely arranged and separated by a considerable amount of connective tissue between them.
Figure 1 shows that the control group rats exhibited a follicular lumen full of colloid and almost no restorative vesicles. In comparison, Figure 2 shows that most of the follicular luminal cavities were empty, and only scanty colloid was observed in the lumina of follicles, particularly in experimental groups. Abundant receptive follicles were also observed in the follicular lumina of experimental group rats.
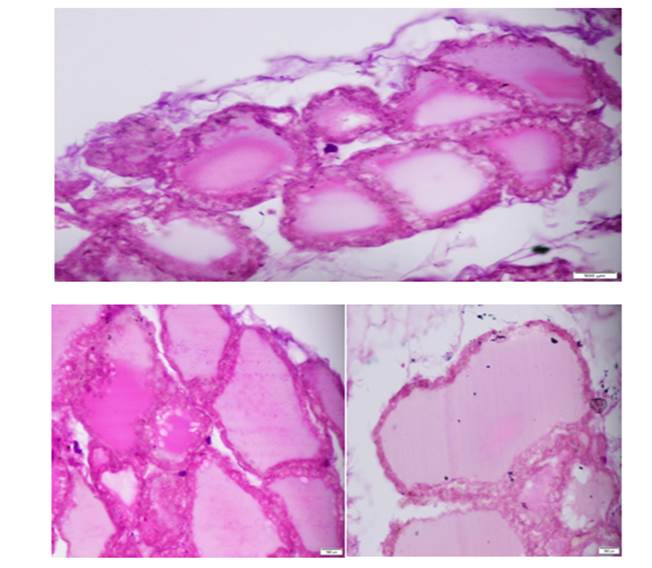
Figure 1: Photomicrographs from control group A, showing thyroid follicles of rats at 40x
Note large sized colloid filled follicles with intact basement membrane and cuboidal epithelium
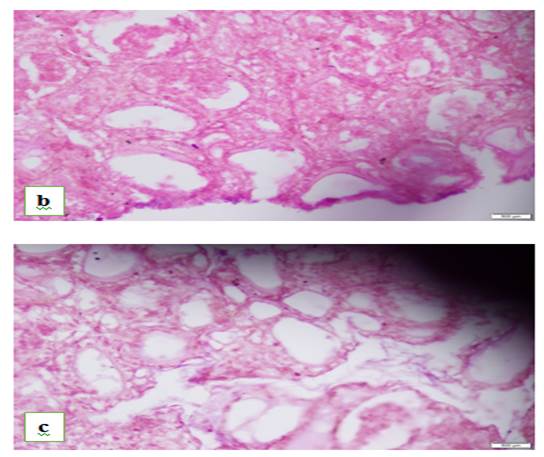
Figure 2: Photomicrographs a and b, representatives of experimental groups B and C respectively showing thyroid follicles of rats at 40x objective
Group B: Note small-sized thyroid follicles with scanty colloid, Group C: Note abundant resorptive vacuoles in colloid with disrupted thyroid follicles
A few par follicular cells were also visible lying within the basement membrane of follicles, and some were scattered in the connective tissue of par follicular space. Darkly stained cells in the connective tissue of par follicular space were identified and characterized as inflammatory infiltrates. This inflammatory infiltrate was abundantly seen in the experimental group rats.
DISCUSSION
Results indicated that rats exposed to the variable duration of loud noise stress in experimental groups B and C exhibited significantly higher follicular epithelial height and reduced follicular diameter than the control group rats. However, the thyroid gland histological parameters didn’t differ significantly among the experimental groups.
The histopathological evaluation of the thyroid gland in the current study is also consistent with previous rodent research where the thyroid gland of rats exposed to acute noise stress showed an increased number of full follicles among the rats exposed to chronic noise stress.18 In a different study, prolonged stress exposure in rodents also lowered serum T3 and TSH, raised rT3, and had no effect on serum T4.19 Thyroid hormone levels was not performed in our study because there were already many studies conducted which focused on the biochemical aspect and measured them. So, there was no gap in literature which needed to be filled by checking their levels again.
There is some evidence of altered thyroid gland functioning in human cohorts exposed to various stress sources.20, 21 People who underwent extensive intraperitoneal surgery stress, for instance, showed decreased free and total T3 levels, increased r T3, free T4, and TSH. People who experienced prolonged posttraumatic stress showed reduced thyroid axis function and increased production of rT3.19 However, there is currently no data on histomorphological variations of the thyroid gland specifically among humans exposed to loud noise stress, hindering standardized comparison and generalization.
The effects of thyroid malfunction brought on by stress have also been verified by studies on other species. For instance, after exposure to chronic stress, hypothyroidism, which is manifested by decreased free T3 levels and elevated TSH levels, has been found in storks, cockerels, newts (Trituruscarnifex), turtles, and fish (Oreochromisniloticus).19 The findings of the present study point to an overactive thyroid gland in response to noise stress exposure, which may perhaps occur before the onset of the hypothyroidism seen in other investigations. Chronic loud noise stress exposure may eventually cause thyroid follicular fatigue and hypo function.
Given that the current study's findings align with data from relevant research in other experimental models, it is crucial to conduct standardized observational and experimental studies among human cohorts exposed to loud noise stress to draw convincing conclusions. While several mechanisms of stress-induced thyroid dysfunction have been proposed, a definitive understanding of the responsible path physiological mechanism is yet to be elucidated.
The qualitative histological findings of our study showed increased height of follicular epithelial cells and decrease in the diameter of thyroid follicles of experimental groups, The study results are consistent with the results in a similar study which observed tall columnar cells with scanty colloid and hence, indicating the hyperactivity of the thyroid gland. 22
It is hypothesized that thyroid deregulation in rats subjected to chronic stress is brought on by impaired T3 negative feedback to TSH brought on by glucocorticoids.19 The concurrent feedback inhibition of CRH and proTRH mRNA caused by glucocorticoids in the paraventricular hypothalamic nucleus is one example of how epigenetics may contribute to thyroid gland dysfunction. 12 The above-given effects of stress-induced thyroid dysfunction are also proposed to be contributed by noise-induced oxidative stress i.e., the imbalance between oxidative and antioxidant metabolites in the body. The similar histological findings in our study were observed by Studer who concluded that it is due to rapid cellular multiplication under stress to meet the needs of the gland under stress. 23
The current study's findings have brought attention to the hyperactivity of thyroid follicles in response to loud noise stress. The outcomes did not significantly differ between experimental groups subjected to noise stress for varying lengths of time. This study is, however, limited to the histopathological evaluation of the thyroid gland. Still, the results thus produced can trigger further research to broadly explore the path physiological basis along with histoarchitecture of the thyroid gland e.g., thyroid serum profile and oxidative stress markers. Further standardized observational and experimental research with human cohorts exposed to loud noise stress should be carried out in order to draw conclusions and generalize these findings.
CONCLUSION
Exposure to variable durations of loud noise stress in adult rats leads to histomorphological changes in the thyroid gland, including increased follicular epithelial height and reduced follicular diameter. These results raise the possibility that thyroid follicles may become hyperactive in response to noise stress. Further research, including standardized human studies, is warranted to better understand the underlying mechanisms and potential implications for human thyroid health.
REFERENCES
1.Selye H. A syndrome produced by diverse nocuous agents. Nature 1936;138(3479):32-.
https://doi.org/10.1038/138032a0
2. Liu F, Jiang S, Kang J, Wu Y, Yang D, Meng Q, et al. On the definition of noise. Humanit Soc Sci Commun 9, 406 (2022). https://doi.org/10.1057/s41599-022-01431-x
3. Garcia Ruiz A, South N. Surrounded by sound: Noise, rights and environments. Crime Media Culture 2019;15(1):125-41. https://doi.org/10.1177/1741659017751223
4. Neitzel RL, Fligor BJ. Risk of noise-induced hearing loss due to recreational sound: Review and recommendations. J Acoustical Soc Am 2019;146(5):3911-21. https://doi.org/10.1121/1.5132287
5. World Health Organization. Environmental noise guidelines for the European region. 2018.
6. Luca M, Guzik T, Luca A. Oxidative Stress as a Link between Cerebrocardiovascular and Psychiatric Disorders. Oxidative Med Cellular Longevity. 2020;2020. https://doi.org/10.1155/2020/5685317
7.Zaman M, Muslim M, Jehangir A. Environmental noise-induced cardiovascular, metabolic and mental health disorders: A brief review. Environ Sci Poll Res 2022;29(51):76485-500. https://doi.org/10.1007/s11356-022-22351-y
8. Hall JE, Hall ME. Guyton and Hall textbook of medical physiology e-Book. 14th ed. Philadelphia: Elsevier Health Sciences; 2020
9. Barrett KE, Boitano S, Barman SM, Brooks HL. Ganong’s review of medical physiology 26 ed. New York: MC Graw Hill; 2019 Dec 29. page 337-50
10. Chiovato L, Magri F, Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. AdvTher 2019;36:47-58. https://doi.org/10.1007/s12325-019-01080-8
11.Bolm-Audorff U, Hegewald J, Pretzsch A, Freiberg A, Nienhaus A, Seidler A. Occupational noise and hypertension risk: a systematic review and meta-analysis. Int JEnviron Res Public Health 2020;17(17):6281. https://doi.org/10.3390/ijerph17176281
12. Leso V, Fontana L, Finiello F, De Cicco L, Ercolano ML, Iavicoli I. Noise induced epigenetic effects: A systematic review. Noise Health 2020;22(107):77-89https://doi.org/10.4103/nah.NAH_17_20
13. Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: A review. EXCLI J. 2017;16:1057. https://doi.org/10.17179/excli2017-480
14. Veljović V, Jovanović J, Radević L, Radovanović Z, Gligorijević S, Blagojević L. Early detection of thyroid disease in workers professionally exposed to noise through preventive medical check-ups. Acta Medica Medianae 2010;49(3):45-8
15. Shomer NH, Allen-Worthington KH, Hickman DL, Jonnalagadda M, Newsome JT, Slate AR, et al. Review of rodent euthanasia methods. J Am Assoc Lab Anim Sci 2020;59(3):242-53. https://doi.org/10.30802/AALAS-JAALAS-19-000084
16. Sakhvidi MJ, Sakhvidi FZ, Mehrparvar AH, Foraster M, Dadvand P. Association between noise exposure and diabetes: A systematic review and meta-analysis. Environ Res 2018;166:647-57. https://doi.org/10.1016/j.envres.2018.05.011
17.Ntumi S. Reporting and interpreting One-Way Analysis of Variance (ANOVA) using a data-driven example: A practical guide for social science researchers. J Res Educ Sci (JRES) 2021;12(14):38-47. https://doi.org/10.14505/jres.v12.14.04
18.Ababzadeh S, Razavinia F-S, Farsani ME, Sharifimoghadam S, Moslehi A, Faghani D. Effect of short-term and long-term traffic noise exposure on the thyroid gland in adult rats: a sexual dimorphic study. Hormone Mol Biol Clin Invest 2021;42(1):29-35.https://doi.org/10.1515/hmbci-2020-0029
19. Nadol'nik LI. [Stress and the thyroid gland]. Biomed Khim 2010;56(4):443-56.
20. https://doi.org/10.1134/S1990750811020119
21.Themann CL, Masterson EA. Occupational noise exposure: A review of its effects, epidemiology, and impact with recommendations for reducing its burden. JAcoustical Soc Am 2019;146(5):3879-905. https://doi.org/10.1121/1.5134465
22.Kurien MJ, Rajagopalan A, Sailesh KS, Varghese V, Amin A, Reddy UK, et al. Stress, thyroid hormone secretion and vestibular stimulation: a review of the links. Bio, Engin, Med Sci Rep 2015 Jul 1;1(2):31-4.https://doi.org/10.5530/BEMS.1.2.1
23. Eaton JL, editor. Thyroid disease and reproduction: a clinical guide to diagnosis and management: Springer; 2018 Oct 31
24.Studer H, Derwahl M. Mechanisms of nonneoplastic endocrine hyperplasia—a changing concept: a review focused on the thyroid gland. Endo Rev 1995;16(4):411-26. https://doi.org/10.1210/edrv-16-4-411
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work. GRANT SUPPORT AND FINANCIAL DISCLOSURE The study is supported by the grant from the National University of Medical Sciences (NUMS), Islamabad, Pakistan |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |