![]() https://doi.org/10.35845/kmuj.2024.23508
ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2024.23508
ORIGINAL ARTICLE
Efficacy of simulated equestrian therapy in improving gait parameters among children with Down syndrome: a randomized controlled trial
Maha Siddiqui 1, Sumaira Farooqui 1 ![]() , Jaza Rizvi 1,
Bashir Ahmed Soomro 2
, Jaza Rizvi 1,
Bashir Ahmed Soomro 2
|
1: Ziauddin College of Rehabilitation Sciences, Ziauddin University, Karachi, Pakistan 2: Department of Neurology, Ziauddin Hospital, Karachi, Pakistan
Email Contact #: +92-300- 2375813 Date Submitted: October 08, 2023 Date Revised: December 22, 2023 Date Accepted: December 28, 2023 |
|
THIS ARTICLE MAY BE CITED AS: Siddiqui M, Farooqui S, Rizvi J, Soomro BA. Efficacy of simulated equestrian therapy in improving gait parameters among children with Down syndrome: a randomized controlled trial. Khyber Med Univ J 2024;16(1):38-44. https://doi.org/10.35845/kmuj.2024.23508 |
ABSTRACT
OBJECTIVE: To compare the effectiveness of Simulated Equestrian Therapy (SET) and Standard Physical Therapy (SPT) in improving gait parameters among children with Down syndrome (DS).
METHODS: This single-blinded, randomized controlled trial was conducted at Dar-ul-Sukun Institute and Dr. Zaiuddin Hospital, Karachi, Pakistan, from April to August 2023. Sixty individuals, meeting the selection criteria were randomly assigned to either the treatment group (n=30) receiving SET or the control group (n=30) undergoing SPT. Nevertheless, two participants from each group either discontinued the treatment or failed to comply with the therapy and 56 participants (n=28 in each group) were included for analysis. Safety measures included recording blood pressure and heart rate before and after each session. Temporal gait parameters were assessed using the 10-Meter Walk Test at baseline, 6 weeks, and 12 weeks. Data was analyzed using Medical software.
RESULTS: Temporal gait parameters were analyzed for 56 (33 males and 23 females) children with DS in both groups. Mean age of patients in SET & SPT groups was 7.89±1.7 and 8.07±0.76 years respectively. Baseline showed similar scores in both groups. At 6 weeks, SET demonstrated significant improvement in cadence (1.78 SPM to 1.35) and gait velocity (2.11 MPS to 2.79), paralleled by SPT (cadence: 2.01 to 1.32 SPM, gait velocity: 2.12 to 2.83 MPS). Between-group analysis revealed no significant differences.
CONCLUSION: Both SET & SPT effectively improve gait parameters in children with Down syndrome. The comparable outcomes highlight SET as a viable alternative, providing clinicians and caregivers additional options for personalized therapeutic approaches.
Clinical Trial Registration Number: NCT05912803
KEYWORDS: Down Syndrome (MeSH); Developmental Disabilities (MeSH); Gait (MeSH); Physical Therapy Modalities (MeSH).
INTRODUCTION
Down Syndrome (DS) is a chromosomal condition prevalent in the pediatric population that imposes significant medical and societal burdens.1 It constitutes roughly 8% of all congenital abnormalities.1,2 DS arises from various causes, including complete trisomy 21 (94%), mosaicism (2.4%), or translocations (3.3%).3 Its main characteristics encompass fluctuating degrees of intellectual disability and a distinctive facial appearance marked by small ears, a flat nose, upward-slanting eyes with small white spots in the irises, a protruding tongue, small hands and feet, and a short neck. Additionally, individuals with DS exhibit short stature, generalized joint laxity, and hypotonia.4-6 Hypotonia and joint laxity contribute to reduced balance and coordination, delaying the development of gross motor skills. These factors also give rise to musculoskeletal issues and misalignment in the lower extremities, leading to inefficient and abnormal movement patterns that compromise mobility and daily functioning.
Consequently, this population’s challenges are exacerbated, and the vicious circle of difficulties continues.3,7,8 As mentioned earlier, the cumulative effects of these factors place considerable stress on the feet, altering the gait pattern in these individuals.3 People with DS exhibit a unique gait pattern often called chaplain’s gait, characterized by a slow walking speed, small step length, reduced cadence and larger step width.9
Children with DS face considerable motor development challenges, including reduced coordination, precision, and effectiveness in movement compared to typically developing peers. These difficulties manifest in awkward, uncoordinated movements and a limited ability to coordinate multiple joint movements.10 To address these challenges, children with DS often engage in various rehabilitative therapies, with physical therapists playing a crucial role within the multidisciplinary care team through early intervention and the prevention of future complications.11 Despite the necessity of these interventions, current literature points to a significant issue with the DS population's adherence to traditional long-term therapies. This highlights the critical need for more engaging and enjoyable therapeutic approaches. Simulated Equestrian Therapy, a play-based intervention that utilizes an exercise toy, offers an innovative solution by actively involving children with DS in their treatment, potentially making the process more enjoyable and effective. This study was planned to explore the impact of Simulated Equestrian Therapy on improving gait patterns in children with DS.
Methods
Sample, Design and Settings
This single-blinded, randomized-controlled trial was conducted in the Department of Rehabilitation of Dar-ul-Sukun Institute and Dr. Ziauddin Hospital, Karachi, Pakistan. This research was carried out in accordance to the Declaration of Helenski. The ethical review for this trial was obtained from the Ziauddin University ERC committee under reference code #6803223MHREH, dated March 14, 2023.
The sample size was determined to be 48 participants, divided equally into two groups, to achieve a 95% confidence level and 80% power, considering a 70% anticipated outcome rate and a 5% margin of error.12
In this study, 62 participants were screened, and from this pool, 60 individuals meeting the selection criteria were randomly assigned to either the treatment group (n=30) receiving Simulated Equestrian Therapy (SET) or the control group (n=30) undergoing standard physical therapy (SPT). The random allocation was facilitated by employing a simple random sampling technique with the use of sealed envelopes. Consent was obtained from the parents, and assent was secured from the subjects. Nevertheless, two participants from each group either discontinued the treatment or failed to comply with the therapy. In the end, 56 participants (n=28 in each group) were included for analysis (Figure 1).
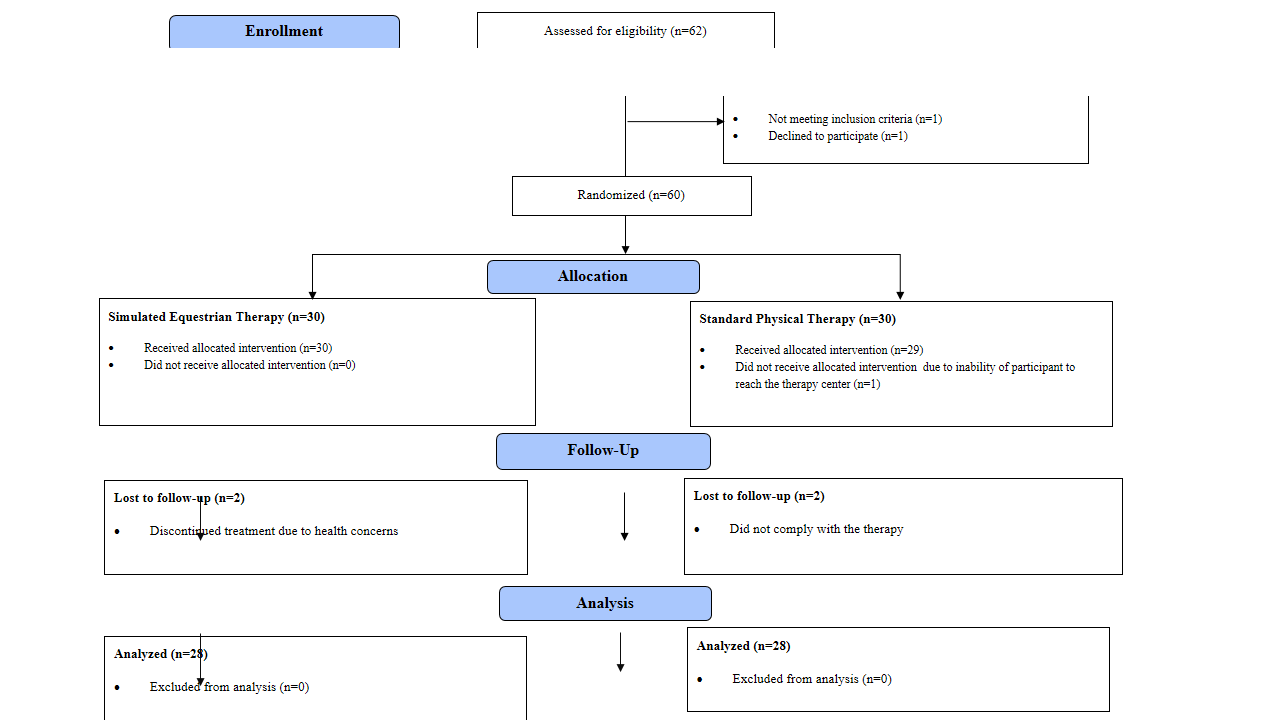
Figure 1: CONSORT Flow Diagram
The participants and their guardians were blinded to the group allocations. Individuals referred by a physician diagnosed with DS based on characteristic features, aged 6–12 years, and having a gross motor function classification (GMFCS) of level I were included. The child's motor skill status, assessed through the standardized GMFCS questionnaire, determined their GMFCS level at the screening. Those participants who already had a similar intervention within the last year or had any atlantoaxial instability, behavioral, cognitive or severe visual impairment were excluded from the study.
Pre-requisites for Intervention
Each participant’s blood pressure (BP) and heart rate (HR) were recorded before and after each session to ensure safety. Those participants, who failed to achieve regular resting readings as defined by the American Heart Association or felt uncomfortable, even after rest were provided with a compensatory session, and their scheduled session was cancelled. Each participant was assessed at baseline after 6 and 12 weeks of intervention using the 10-meter walk test (10MWT).17 Each group received the treatment three times a week for three months. Each session’s duration was 30-45 minutes on average, which varied with the weekly progression.
Intervention Group
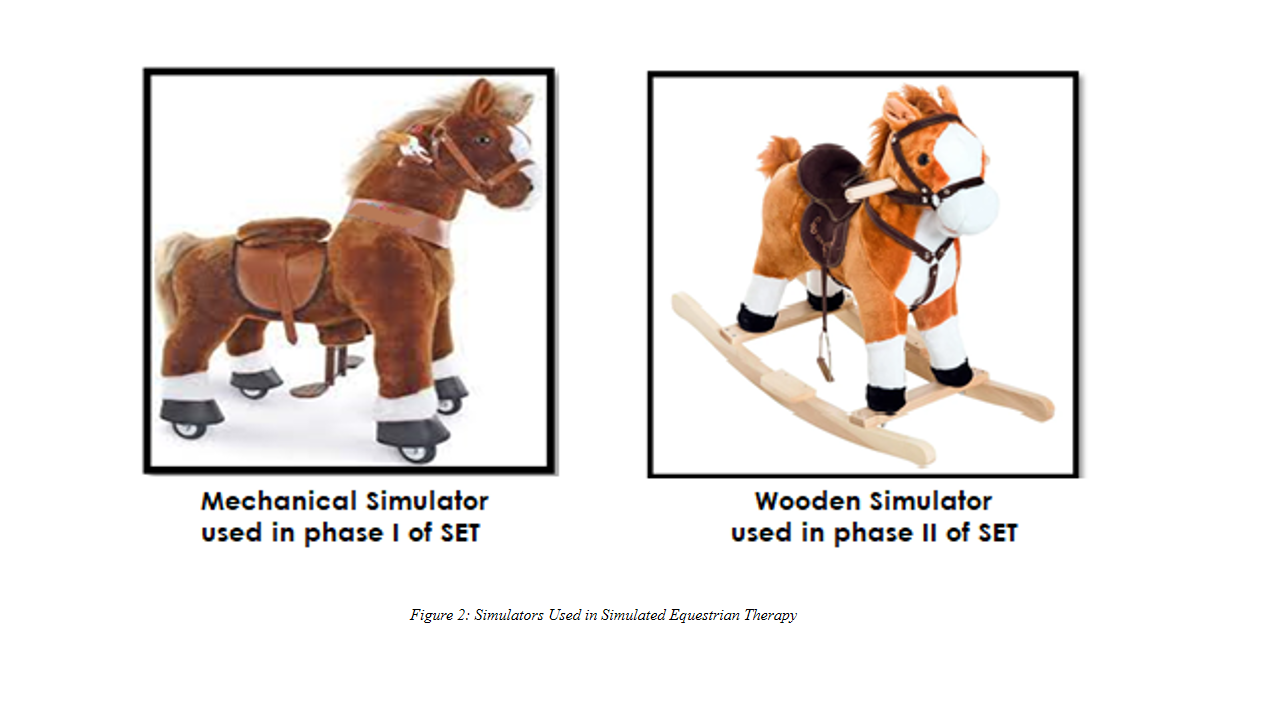
SET was administered in two phases under the supervision of an experienced physical therapist, utilizing two horse simulators (mechanical and wooden), as illustrated in Figure-2.
Protocol for SET
· Warm-up: To stimulate the vestibular and proprioceptive senses, the child performed a swinging motion on the wooden rocking horse simulator for 5 minutes in an anterior and posterior direction as a warm-up. This pattern mimicked the rhythmic movement of a horse’s pelvis to provide a near-realist experience to the rider.
· Phase-I: This phase aimed to incorporate the horse gait movements of trot and gallop that are experienced while riding a real horse and to stimulate and strengthen the muscles, including the Deltoid, Bicep, Triceps, Hamstrings, Calf, Quadriceps, Latissimus Dorsi, Abdominals, and Back Extensors of the participant.22 Unlike real Equestrian Therapy settings, the treatment environment was controlled to ensure the child’s safety. Using measurement tape and markers, a block 10 feet in length and 5 feet in breadth was constructed in the treatment area for exercising in this phase. The child was asked to complete four rounds around this custom-built block while riding the mechanical walking horse. A rest period of 5 minutes was kept to maintain the participants’ energy levels, which they utilized either in between or after this phase.
· Phase II: This phase focused on enhancing the child's motor performance by stimulating and coordinating their vestibular, proprioceptive, and neuro-muscular systems. Adapted from Champagne, Corriveau, and Dugas,13 the child engaged in goal-oriented activities on a wooden rocking horse simulator, performing 8-12 repetitions each in forward, backward, and lateral directions. Exercise specifics are outlined in Table I.
· Cool-down: This phase was followed by the exercise phase, which consisted 5 minutes of anterior and posterior swinging on the wooden simulator and deep breathing exercises.
Control Group
Protocol for SPT
· Warm-up: In the initial warm-up phase, the child sat on a therapy ball for 5 minutes and swinging in an anterior and posterior direction was performed to mimic the rhythmic movement of a horse’s pelvis.
· Training Protocol: This phase was adapted from Ghafar and Abdelraouf.14 It included overall stability and body balancing exercises performed in a controlled indoor environment to ensure the safety of the participants, strengthen the core and develop the coordination and balance required for task performance. Each activity was performed in a set of 2-3 with 8-12 repetitions. Details of the exercises are listed in Table-1.
· Cool-down: It was followed by the training phase, comprised of 5 minutes of swinging on a therapy ball in an anterior and posterior direction and deep breathing exercises.
Table I: Weekly Progression of Exercises in SET and SPT group
|
Weeks |
Exercise plan for SET (Phase-II) |
Exercise Plan for SPT |
|
0-2 |
· Practicing catching and throwing |
· Practicing throwing and catching balls outside of their base of support · Walking on a 5 cm thick balance beam of 1 yard |
|
3-5 |
· Placing the ball, and rings on the target
|
· Maintenance of balance over a tilt board during sitting, standing, and squatting positions for 3-5 minutes each |
|
6-8 |
· Performing target hitting on a game of dart
|
· Walking up and down stairs to collect objects · Passing over 5 cm obstacles like cones and foam blocks |
|
9-12 |
· Stretching to the head, feet, and tail of the horse |
· Maintenance of stability by unilateral standing, alternatively, with eyes open for 10 seconds to 1 minute · Kicking and jumping activities |
Outcomes and Measuring Parameters
Outcomes included 2 parameters of gait: cadence and gait velocity. Cadence is the number of steps a person takes in a minute.15 One of the most important, accessible, and measurable parameters of gait and gait velocity is the speed of an individual while walking.16 The outcome assessors measured the outcomes using the 10 MWT, blinded to the participant’s allocation. The description of data collection using the outcome measure is as follows:
- 10 MWT has an excellent reliability of r=0.91.17
- For the testing, a straight line of 10 meters was drawn on the ground with the help of measuring tapes and markers.
- The line had two zones marked as acceleration and deceleration to record the walking time of the participants.
- The participants were then asked to comfortably walk, at their usual speed, on a line following verbal cues.
- A stopwatch was used to record the time, and steps were counted.
- Incentives in candies, toys, and signaling cues were provided to the children who froze between the tests or could not follow verbal instructions.
- Before the incorporation of testing, all the children were oriented from the testing environment to reduce their fear, confusion and agitation.
- Testing was performed in a separate room to eliminate the environment’s effects on the child’s performance.
Statistical Analysis
The data was analyzed using Medical software. ‘Skewness and Kurtosis Rule of Thumb’ was applied to test the normality of the data. Since the data was found to be skewed, ‘Friedman’s ANOVA’ and ‘Mann-Whitney U Test’ for within and between the groups analysis were applied. Descriptive statistics are reported in terms of mean and standard deviation, whereas continuous variables are displayed as median (25th to 75th percentile, lowest to highest) and p-value (<0.05) considered significant.
RESULTS
This study included 56 children with DS, comprising 33 males and 23 females, assigned to SET and SPT groups. Mean age of patients in SET & SPT groups was 7.89±1.7 years and 8.07±0.76 years respectively (Table-II). Temporal gait parameters, including gait velocity and cadence, were assessed at baseline, after the 6th and 12th weeks of intervention.
Table II: Demographic details of participants
|
Variables |
Group |
N |
Mean ± S.D |
Normality |
|
Age (Years) |
SET |
28 |
7.89±1.7 |
Reject Normality |
|
SPT |
28 |
8.07±0.76 |
Reject Normality |
|
|
Height (cm) |
SET |
28 |
136.50±7.43 |
Accept Normality |
|
SPT |
28 |
134.25±3.59 |
Reject Normality |
|
|
Weight (kg) |
SET |
28 |
51.21±7.13 |
Accept Normality |
|
SPT |
28 |
51.14±2.10 |
Accept Normality |
SET: Simulated Equestrian Therapy; SPT: Standard Physical Therapy; S.D: Standard Deviationp<0.001* considered as highly significant; SET: Simulated Equestrian Therapy; SPT: Standard Physical Therapy
Within-Group Analysis
Baseline Comparison:
At baseline, both the groups, SET and SPT, had similar scores.
Baseline to 6th Week:
In the SET group, cadence mean rank decreased from 2.85 to 1.78 steps per minute (SPM), and gait velocity increased from 1.09 to 2.11 meters per second (MPS) in the 6th week of intervention, showing a significant performance improvement (p <0.001). While, in the SPT group, there was a significant change in cadence from 2.66 to 2.01 SPM and gait velocity from 1.03 to 2.12 MPS, with p < 0.001.
6th to 12th Week:
The SET group exhibited a decrease in the mean rank of 1.78 to 1.35 SPM in the parameter of cadence and an increase in the mean rank of 2.11 to 2.79 MPS for gait velocity, indicating significant differences p <0.001. Likewise, effective results were reported in the SPT group, where the mean rank in cadence (2.01 to 1.32 SPM) and gait velocity (2.12 to 2.83 MPS) were also remarkable with a value of p <0.001.
Between-Group Analysis
The pair-wise comparison results indicated no significant differences between SET and SPT groups during different intervention points, as indicated by the constant value of Hodges-Lehmann median difference for cadence (-2.0000) and gait velocity (0.0000). The intention-to-treat analysis was applied to reduce potential bias in treatment effects due to attrition rate. Details of the analysis are listed in Table IV.
Table IV: Gait Changes in Down Syndrome Children: Between-Group Comparison of Simulated Equestrian Therapy vs. Standard Physical Therapy
|
Variables |
Lowest value |
Highest value |
Median |
Hodges-Lehmann median difference |
Mann-Whitney U |
Two-tailed probability |
|||||||
|
SET |
SPT |
SET |
SPT |
SET |
SPT |
SET |
SPT |
SET |
SPT |
SET |
SPT |
||
|
Cadence |
Baseline |
43 |
72 |
60 |
53 |
50 |
-2.0000 |
315.50 |
P = 0.2091 |
||||
|
6th Week |
42 |
39 |
70 |
52 |
50 |
328.50 |
P = 0.2969 |
||||||
|
12th Week |
41 |
39 |
70 |
52 |
49 |
318.00 |
P = 0.2242 |
||||||
|
Gait Velocity |
Baseline |
0.12 |
0.20 |
0.17 |
0.16 |
0.0000 |
332.00 |
P = 0.4320 |
|||||
|
6th Week |
0.13 |
0.13 |
0.24 |
0.18 |
0.18 |
347.50 |
P = 0.4589 |
||||||
|
12th Week |
0.13 |
0.13 |
0.25 |
0.19 |
0.19 |
370.50 |
P = 0.7199 |
||||||
SET: Simulated Equestrian Therapy; SPT: Standard Physical Therapy
DISCUSSION
Acquisition of proper gait patterns is of paramount importance for children diagnosed with DS. In addition to encountering delays in cognitive, psychosocial, developmental milestones, and motor skills development, children with DS also experience a compromised gait pattern that impacts their day-to-day activities. Hence, this study was conducted on 56 children with DS to evaluate the effects of SET compared to SET in improving gait parameters. The results analyzed two temporal components of gait, velocity and cadence, using 10MWT and exhibited significant differences in baseline and post-intervention values. Baseline showed similar scores in both groups. Notably, SET demonstrated a significant improvement in cadence and gait velocity (1.09 to 2.11 MPS) at the 6th week, sustained at the 12th week (cadence: 1.78 to 1.35 SPM, gait velocity: 2.11 to 2.79 MPS), with p <0.001. SPT group also exhibited significant changes. Between-group analysis indicated no significant superiority of one therapy over the other.
This study analyzed participants’ performance at three intervals (baseline, 6th and 12th weeks). Each gait parameter was discussed separately to highlight the independent impact of SET and SPT on them. While searching for comparative literature, the authors identified a considerable gap in the research depository for gait parameters utilizing similar treatment approaches, especially for children with DS; however, studies evaluating other spatial and temporal parameters were available.
Numerous researchers have studied gait parameters, and one among them is Sutherland et al.18 who identified parameters of gait maturity in typically developing children and concluded that changes in gait over time in an individual are evident through its five spatiotemporal parameters, including gait velocity, step length, cadence, and duration of single-limb stance and ratio of pelvic span to ankle speed. Hence, the maturity of gait in DS can also be studied by analyzing these variables. Naito et al.19 claim that children with DS exhibit lower values of these parameters compared to peers of the same age; however, they argue that these parameters should improve with advancing age in DS. This study analyzed cadence and gait velocity amongst the given parameters of Sutherland and determined that cadence and gait velocity are inversely proportional to each other. Hence, in a child with DS, a decrease in cadence should result in an increased gait velocity, and our study has significantly supported this claim. Our findings are consistent with Kim et al. who investigated the effects of a three-dimensional horse riding simulator with and without virtual reality training gaming on ten children with cerebral palsy and reported significant changes in all gait parameters, including gait velocity in the horse riding simulator group (pre 0.56±0.13, post 0.61±0.11) as well as the other group.19,20 Current findings are also related to the claims of Moriello et al. who studied the gait parameters in four children with DS following a combination of Equestrian therapy with physical therapy and found significant changes in gait speed.21 Similarly, Portaro et al. studied fifteen males with DS following a 6-month Equestrian therapy protocol and reported a significant improvement in gait speed (pre 0.74±0.1, post 0.89±0.12).22 Since our findings are similar to the studies that utilized real horse riding as a treatment, this protocol can be considered an adequate replacement for actual Equestrian Therapy in children with DS. Our findings are also related to Mutoh et al.23 who studied the effects of Equestrian therapy on 24 children with CP and reported significant results in the improvement of both cadence (pre 79.3±28.8, post 104.4±24.3) and gait velocity (pre 31.9±10.7, post 38.8±13.5) and Manikowsa et al.24 who studied 16 ambulatory cerebral palsy children and reported significant findings of improvement in cadence and gait velocity after a single session of Equestrian therapy. These findings make our protocol a considerable asset for children with disabilities, and thus, it can be applied to different developmental disabilities and can significantly contribute to their betterment.
Literature also suggests that anthropometric differences between genders predispose men to have a higher gait velocity and lesser cadence than women. Such differences have also been reported for DS.25 However, our study neither significantly supports this claim nor observed gender-dependent variability of the spatiotemporal parameters. Moreover, the surfacing literature mentioning growth curves in DS advocates that these children attain motor function at a slower pace than their age-matched typically developing peers, which can lead to complications with progressing age. However, early interventions can combat this problem and significantly contribute to DS age-appropriate motor growth.26 Literature also suggests that impaired children’s recreational and exercise needs should be met through play-based therapies.27 Such approaches should be considered an essential component of a child’s care plan; hence, this protocol can be considered a valuable addition.
Strengths and Limitations
Previous studies have been conducted on youth and adolescents with DS using Simulated Equestrian therapy; however, to the author’s knowledge, no study on DS children was conducted; hence, our study is the first of its kind in evaluating gait parameters among DS children, which adds to the scarce literature. It will also serve as a basis for future research where this protocol can be generalized to a more significant sample, and a comparison between genders and age groups could be made. Despite the rigor, it has some limitations, too, including the absence of a follow-up period, which could have provided valuable insight into the long-term effectiveness of the protocols. Moreover, this study did not evaluate changes on the cellular level, which are essential to note in conditions like DS to contribute significantly to the betterment of this community. Furthermore, the induction of children with DS without stratification of its types could also raise a question on the application of this intervention, as the effectiveness can vary with the type of DS.
CONCLUSION
This study suggests that both simulated equestrian therapy and standard physical therapy are effective in improving gait parameters among children with Down syndrome. The comparable outcomes highlight the potential of simulated equestrian therapy as an alternative and potentially beneficial intervention, offering clinicians and caregivers additional options for tailoring therapeutic approaches based on individual preferences and needs. Studies with a follow-up period should be conducted to further evaluate the long-term benefits of SET.
ACKNOWLEDGEMENTS
We thank the participants and their guardians for making this study possible. We would also like to thank the facility of Dar-ul-Sukun and Dr. Ziauddin Hospital for their support and compliance.
REFERENCES
1. Kazemi M, Salehi M, Kheirollahi M. Down syndrome: Current status, challenges and future perspectives. Int J Mol Cell Med 2016;5(3):125-33.
2. Lanzoni M, Kinsner-Ovaskainen A, Morris J, Martin S. Socio-economic regional microscope series - EUROCAT – Surveillance of congenital anomalies in Europe: epidemiology of Down syndrome 1990-2014. Publications Office of the European Union, Luxembourg, 2019, ISBN 978-92-76-00574-2, https://doi.org/10.2760/70796,JRC115824
3. Foley C, Killeen OG. Musculoskeletal anomalies in children with Down syndrome: an observational study. Arch Dis Child 2019;104(5):482-7. https://doi.org/10.1136/archdischild-2018-315751
4. Azfar M, Khan I, Iqbal N, Khawar N, Abid K. Oral health of individuals with down syndrome in Karachi, Pakistan. J Pak Dent Assoc 2018;27(4):190-4. https://doi.org/10.25301/JPDA.274.190
5. World Health Organization. WHO-Genes and Human Disease;2018. [Accessed on: May 10, 2023]. Available from URL: https://www.who.int/genomics/public/geneticdiseases/en/index2.html
6. Lim PQ, Shields N, Nikolopoulos N, Barrett JT, Evans AM, Taylor NF, et al. The association of foot structure and footwear fit with disability in children and adolescents with Down syndrome. J Foot Ankle Res 2015;8(1):4. https://doi.org/10.1186/s13047-015-0062-0
7. Carter K, Sunderman S, Burnett SW. The effect of vestibular stimulation exercises on balance, coordination, and agility in children with Down syndrome. Am J Psychiatry Neurosci 2018;6(2):28-32. https://doi.org/10.11648/j.ajpn.20180602.11
8. Weber A, Martin K. Efficacy of orthoses for children with hypotonia: A systematic review. Pediatr Phys Ther 2014;26(1):38-47. https://doi.org/10.1097/PEP.0000000000000011
9. Zago M, Duarte NAC, Grecco LAC, Condoluci C, Oliveira CS, Galli M. Gait and postural control patterns and rehabilitation in Down syndrome: a systematic review. J Phys Ther Sci 2020;32(4):303-14. https://doi.org/10.1589/jpts.32.303
10. Corsi C, Cimolin V, Capodaglio P, Condoluci C, Galli M. A biomechanical study of gait initiation in Down syndrome. BMC Neurol 2019;19:1-8. https://doi.org/10.1186/s12883-019-1288-4
11. Ruiz‐González L, Lucena‐Antón D, Salazar A, Martín‐Valero R, Moral‐Munoz JA. Physical therapy in Down syndrome: Systematic review and meta‐analysis. J Intellect Disabil Res 2019;63(8):1041-67. https://doi.org/10.1111/jir.12606
12. Pankaj G, Avani K, Salil V. Prevalence of down syndrome in western India: A cytogenetic study. J Adv Med Med Res 2015;5(10):1255-9. https://doi.org/10.9734/BJMMR/2015/13648
13. Champagne D, Corriveau H, Dugas C. Effect of hippotherapy on motor proficiency and function in children with cerebral palsy who walk. Phys Occup Ther Pediatr 2017;37(1):51-63. https://doi.org/10.3109/01942638.2015.1129386
14. Ghafar MA, Abdelraouf OR. Effect of virtual reality versus traditional physical therapy on functional balance in children with down syndrome: A randomized comparative study. Int J Physiother Res 2017;5:2088-94. https://dx.doi.org/10.16965/ijpr.2017.146
15. Tudor-Locke C, Ducharme SW, Aguiar EJ, Schuna JM, Barreira TV, Moore CC, et al. Walking cadence (steps/min) and intensity in 41 to 60-year-old adults: the CADENCE-adults study. Int J Behav Nutr Phys Act 2020;17(1):137. https://doi.org/10.1186/s12966-020-01045-z
16. Middleton A, Fritz SL, Lusardi M. Walking speed: The functional vital sign. J Aging Phys Act 2015;23(2):314-22. https://doi.org/10.1123/japa.2013-0236
17. Shirley Ryan Ability Lab. 10 Meter Walk Test; 2023. [Accessed on: May 10, 2023]. Available from URL: https://www.sralab.org/rehabilitation-measures/10-meter-walk-test
18. Sutherland DH, Olshen RI, Cooper LE, Woo SL. The development of mature gait. J Bone Joint Surg Am 1980;62(3):336-53.
19. Naito M, Aoki S, Kamide A, Miyamura K, Honda M, Nagai A, et al. Gait analysis in Down syndrome pediatric patients using a sheet‐type gait analyzer: Pilot study. Pediatr Int 2015;57(5):860-3. https://doi.org/10.1111/ped.12691
20. Kim HW, Nam KS, Son SM. Effects of virtual reality horse riding simulator training using a head-mounted display on balance and gait functions in children with cerebral palsy: A preliminary pilot study. J Kor Phys Ther 2019;31(5):273-8. https://doi.org/10.18857/jkpt.2019.31.5.273
21. Moriello G, Terpstra ME, Earl J. Outcomes following physical therapy incorporating hippotherapy on neuromotor function and bladder control in children with Down syndrome: A case series. Phys Occup Ther Pediatr 2020;40(3):247-60. https://doi.org/10.1080/01942638.2019.1615601
22. Portaro S, Cacciola A, Naro A, Cavallaro F, Gemelli G, Aliberti B, et al. Can individuals with Down syndrome benefit from hippotherapy? An exploratory study on gait and balance. Dev Neurorehabil 2020;23(6):337-42. https://doi.org/10.1080/17518423.2019.1646830
23. Mutoh T, Mutoh T, Tsubone H, Takada M, Doumura M, Ihara M, et al. Impact of long-term hippotherapy on the walking ability of children with cerebral palsy and quality of life of their caregivers. Front Neurol 2019;10:834. https://doi.org/10.3389/fneur.2019.00834
24. Manikowska F, Jóźwiak M, Idzior M, Chen PJ, Tarnowski D. The effect of a hippotherapy session on spatiotemporal parameters of gait in children with cerebral palsy-pilot study. Ortop Traumatol Rehabil 2013;15(3):253-7. https://doi.org/10.5604/15093492.1058420
25. Pau M, Condoluci C, Zago M, Galli M. Men and women with Down syndrome exhibit different kinematic (but not spatio‐temporal) gait patterns. J Intellect Disabil Res 2019;63(1):64-71. https://doi.org/10.1111/jir.12560
26. Hekal OA, Darwish MM, Attia AAM, Osman ZA, Ahmed I. Effect of selected play activities on adaptive skills among children with down syndrome. Int J Res Appl Nat Soc Sci 2017;5(11):77-86.
27. Palisano RJ, Walter SD, Russell DJ, Rosenbaum PL, Gémus M, Galuppi BE, et al. Gross motor function of children with Down syndrome: creation of motor growth curves. Arch Phys Med Rehabil 2001;82(4):494-500. https://doi.org/10.1053/apmr.2001.21956
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work.
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |