![]() https://doi.org/10.35845/kmuj.2024.23461 ORIGINAL
ARTICLE
https://doi.org/10.35845/kmuj.2024.23461 ORIGINAL
ARTICLE
Role of 6-amino flavone in attenuating cisplatin induced neurotoxicity via inhibition of p-JNK signaling pathway in post-natal day-7 mice
Latafat
Kahkashan Ahmad 1, 2, Rifat Jahan 1 ,Amin Jan 2, 3 ![]() ,
Shahid Ali Shah 2
,
Shahid Ali Shah 2
|
1: Department of Biochemistry, Shaheed Benazir Bhutto Women University, Peshawar, Pakistan 2: Neuro Molecular Medicines Research Center, Department of Biochemistry, Sarhad University of Science and Information Technology, Peshawar, Pakistan 3: Department of Physiology, Northwest School of Medicine, Peshawar, Pakistan
Email
Contact #: +92-333-9400940 Date Submitted: August 30, 2023 Date Revised: January 15, 2024 Date Accepted: January 16, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Ahmad LK, Jahan R, Jan A Shah SA. Role of 6-amino flavone in attenuating cisplatin induced neurotoxicity via inhibition of p-JNK signaling pathway in post-natal day-7 mice. Khyber Med Univ J 2024;16(1):45-51. https://doi.org/10.35845/kmuj.2024.23461 |
ABSTRACT
OBJECTIVE: To investigate the protective role of 6-amino flavone (6-AF) in cisplatin-induced neuro-inflammation in the developing brains of post-natal day-7 (PND-7) mice.
METHODS: This experimental study, conducted at the Neuro Molecular Medicines Research Center in Peshawar, Pakistan, included 20 PND-7 mice from January to March 2023. PND-7 mice were randomly distributed into four groups, a control group, a cisplatin group, a cisplatin + 6-AF group and a 6-AF group. Cisplatin was administered intraperitoneally at a dose of 20 mg/kg to the cisplatin group. 6-AF was injected at a dose of 30 mg/kg after cisplatin administration to cisplatin+6-AF group and 6-AF group mice. After 4 hours of the drug treatment, all the PND-7 mice were sacrificed for Western blot analysis. ImageJ software was used for the densitometry of the blots. One-way ANOVA and post-hoc Tukey tests through Prism Graph-5 were applied for statistical analysis.
RESULTS: Significant differences in p-JNK levels along with TNF-α, NF-κB, and IL-1β proteins were observed in the brain homogenates of PND-7 mice in various groups. A post-hoc Tukey test revealed a significant increase (p<0.001) in the p-JNK, COX-2, NF-κB, and IL-1β levels in the cisplatin group as compared to control group mice. However, a significant decrease (p<0.001) was observed in p-JNK, COX-2, NF-κB, and IL-1β expression levels in the cisplatin + 6-AF group as compared to the cisplatin group.
CONCLUSION: Administration of 6-AF effectively reduced cisplatin-induced neurotoxicity in PND-7 mice, demonstrating a neuroprotective effect by suppressing p-JNK and its downstream TNF-α, NF-κB, and IL-1β proteins.
KEYWORDS: Cisplatin (MeSH); 6-Amino flavone (Non-MeSH); Neuroinflammatory Diseases (MeSH); P-JNK signaling pathway (Non MeSH); NF-κB (Non-MeSH); Tumor Necrosis Factor-alpha (MeSH).
INTRODUCTION
Cisplatin, or cis-diamminedichloroplatinum (II), discovered in the mid-19th century by Michel Peyrone, emerged as a significant chemotherapeutic agent after its biological effects piqued scientific interest in the late 1960s.1 By 1971, cisplatin was undergoing clinical trials for cancer therapy, marking its introduction into the realm of cancer treatment.2 Despite the advent of numerous chemotherapy drugs over the years, cisplatin retains its status as a cornerstone in treating various human malignancies, including sarcomas, carcinomas, lymphomas, and germ cell tumors.3
The therapeutic use of cisplatin is not without adverse effects. Common short-term side effects encompass nausea and vomiting, experienced by virtually all patients undergoing treatment. More severe, dose-dependent side effects include ototoxicity, renal toxicity, hematologic disorders, gastrointestinal issues, and notably, neurotoxicity, manifesting as symptoms ranging from tingling and numbness to loss of proprioception and spinocerebellar ataxia. Notably, cisplatin-induced neurotoxicity represents a major dose-limiting factor, underlining the need for therapeutic strategies to mitigate this challenge.4,5
The oxidative stress induced by high doses of cisplatin contributes significantly to its toxicity profile. Studies in rat brain tissue have demonstrated an increase in pro-oxidants like lipid peroxidation and myeloperoxidase activity, alongside a reduction in antioxidants such as glutathione, highlighting the oxidative damage mechanism.6 The FDA's approval of cisplatin in 1978 underscored its efficacy, making it one of the most potent drugs in the oncological arsenal.7 At the molecular level, cisplatin exerts its anti-cancer effects by crosslinking DNA's purine bases, thereby disrupting DNA repair mechanisms and inducing apoptosis in cancer cells.8
In contrast to cisplatin's toxicological profile, flavonoids—a diverse group of phytonutrients found in fruits, vegetables, flowers, wine, chocolate, and tea, encompassing approximately 9000 identified members—demonstrate antioxidant, anti-inflammatory, antimicrobial, and cardioprotective properties.9 These compounds, including subclasses like flavones, isoflavones, flavanols, flavanonols, and flavanones, have gained attention for their therapeutic potential, particularly in formulations designed to leverage their antioxidant and anti-inflammatory effects.10
Amid this backdrop, the synthetic flavone derivative, 6-aminoflavone (6-AF), exhibits pronounced anti-proliferative activity against MCF-7 breast tumor cells, signaling its potential as a chemotherapeutic agent.11 This study was planned to examine if 6-AF, a derivative of flavonoids, possesses antioxidant and anti-inflammatory capabilities comparable to those observed against potential neuro-inflammation caused by cisplatin treatment in different cancers. Therefore, we aimed to explore the potential of 6-AF in alleviating neuro-inflammation induced by cisplatin in PND-7 mice, investigating its therapeutic efficacy.
METHODS
Healthy adult male and female albino mice (7-8 weeks old and 32 g body weight) were purchased from the Veterinary Research Institute, Peshawar, Pakistan. Mice were randomly distributed in 5 pairs. They were acclimatized to the environment and were provided with food and water ad libitum and a 12-hour light/dark cycle at 25 ±°C. The mice reproduced, and their pups were kept until the 7th postnatal day. A resource equation approach was used for sample size calculation. The sample size was comprised of 20 PND-7 mice. Mice were randomly distributed into four groups, i.e.; a control group (n = 5), a cisplatin group (n = 5), a cisplatin + 6-AF group (n = 5), and a 6-AF group (n = 5). These mice were labeled and kept in separate cages (Biobase China) at the Neuro Molecular Medicines Research Center (NMMRC), Peshawar. A single dose of 20 mg/kg of cisplatin was administered intraperitoneally (IP) to cisplatin group PND-7 mice. On the other hand, 6-mino-flavone was injected at a dose of 30 mg/kg after cisplatin administration to cisplatin + 6-AF group and 6-AF group mice. After 4 hours of cisplatin and 6-AF administration, all the PND-7 mice were sacrificed for laboratory investigations. It was an experimental study performed at NMMRC, Peshawar, Pakistan, from January 2023 to March 2023. Study was approved by the NMMRC animal ethical committee via Ref. No. 11/2022, dated: 05/03/2023.
All animals were sacrificed in accordance with the method described earlier,12 After decapitating the animals, we carefully and promptly removed the brain tissue and stored it on ice in a 1:1 mixture of RNA later solution and phosphate buffer solution (PBS). A total protein extraction solution (T-PER) was used to properly homogenize the brain tissue, which was then collected and kept at -20ºC for future analysis. Absorption at 595 nm was used to measure protein concentration in homogenates using a Bio-Rad protein estimation assay. SDS-PAGE at a concentration of 12–15 percent was used to electrophorese each sample's protein after it had been standardized to 30 mg and 50 mA was used for around 20–30 minutes, then 120 V was used for about 1–1.5 hours till the run was finished. Semi-dry Bio-Rad Transblot technology was used to transfer proteins from gel to poylvinylidene difluoride (PVDF) membranes (Santa Cruz, CA, USA). Various mouse-derived primary antibodies, such as anti-NF-κB (SC-8414), anti-TNF-α (SC-52746), anti-IL-1β (SC-12742), and anti-p-JNK (phospho-c-Jun N-terminal kinase) [SC-6254] manufactured by Santa Cruz Technologies, USA, were employed, while HRP-conjugated secondary antibodies were procured from Promega, Melbourne, USA. The trans-blot results of electrophoresis were developed on X-ray films using chemiluminescence assay reagents.
The original X-ray films of the Western blot analysis were scanned. Image J software was used to perform densitometry analysis of the bands. Integral optical density (IOD) of proteins was expressed in arbitrary units (A. Us) as mean±S.E.M. A significant difference was determined using one-way analysis of variance (ANOVA), followed by a post-hoc Tukey's test on GraphPad Prism 5. A p-value less than 0.05 (P ≤ 0.05) was considered statistically significant.
RESULTS
p-JNK is known as a stress-activated protein kinase. Many stressful conditions cause overexpression of the p-JNK protein, which is actively involved in the mediation of inflammatory and apoptotic signaling. Cisplatin is the most common cause of the activation of phospho-JNK proteins. Therefore, we analyzed the p-JNK level through Western blotting. One-way ANOVA analysis showed significant differences in p-JNK levels in various groups. A post hoc Tukey test revealed that cisplatin caused a significant increase (p<0.001) in the phospho-JNK level in the adult mouse brain. However, 6-AF significantly rectified (p<0.001) phospho-JNK in cisplatin + 6-AF group (Figure 1).
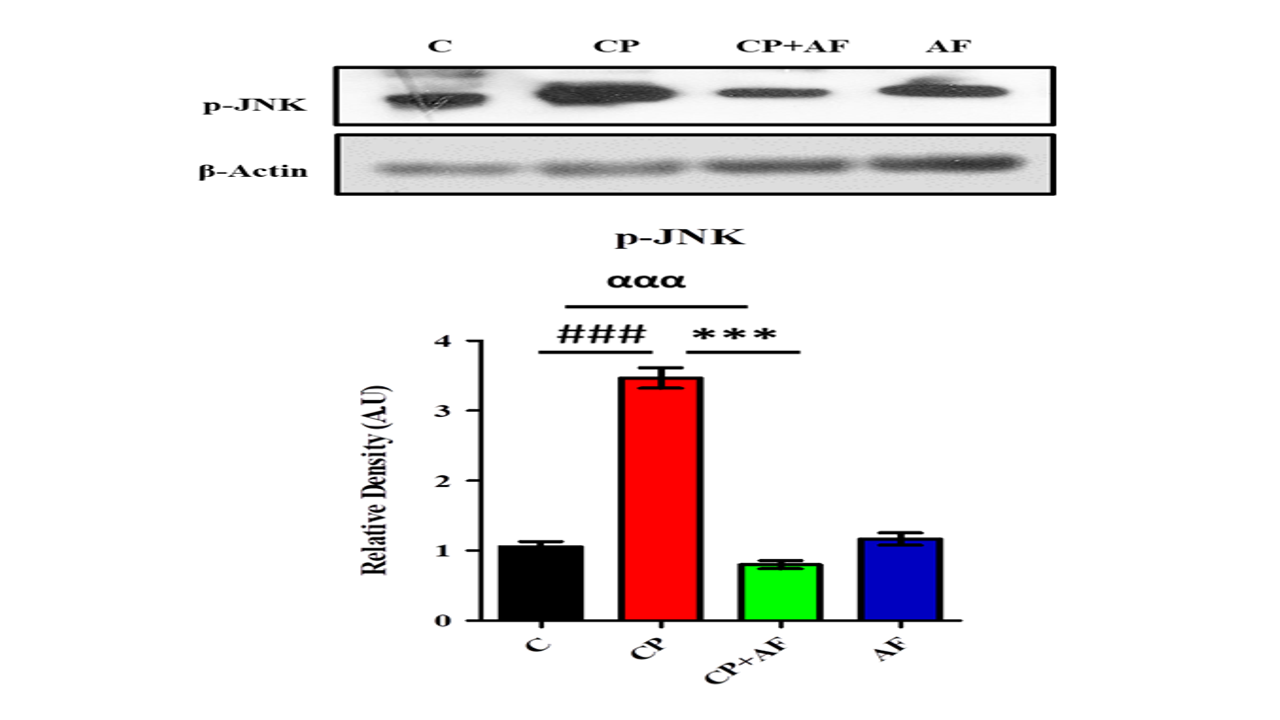
Figure 1: 6-AF successfully ameliorated the expression level of p-JNK protein in the PND-7 mice brains of the cisplatin +6-AF group (shown are the Western blot results of p-JNK expression levels, along with their bar-chart representation in various experimental mouse groups, respectively). β-Actin was used as a loading control. The results were determined using Image J software, and the bar chart indicates the mean in A.U. ± SEM. The significance of a one-way ANOVA is expressed as α, the significance of control vs. cisplatin is expressed as #, and the significance of cisplatin vs. cisplatin + 6-AF is expressed as*. Significance: ***, ###p<0.001).
One-way analysis showed
significant differences (p<0.001) in the expression levels of all the
assessed neuroinflammatory markers, i.e., COX-2, IL-1β, and NF-κB, in the brain
homogenates of mice of various groups. A post-hoc Tukey test revealed that
cisplatin significantly increased (p<0.001) COX-2, IL-1β, and NF-κB levels
in mice brains. However, 6-HF administration significantly inhibited
(p<0.001) the expression of COX-2 (Figure 2), IL-1β (Figure 3), and NF-κB
proteins (Figure 4) in the cisplatin + 6-AF group mice as compared to the
cisplatin group. These findings ascertained 6-AF's role as an anti-neuroinflammatory
agent.
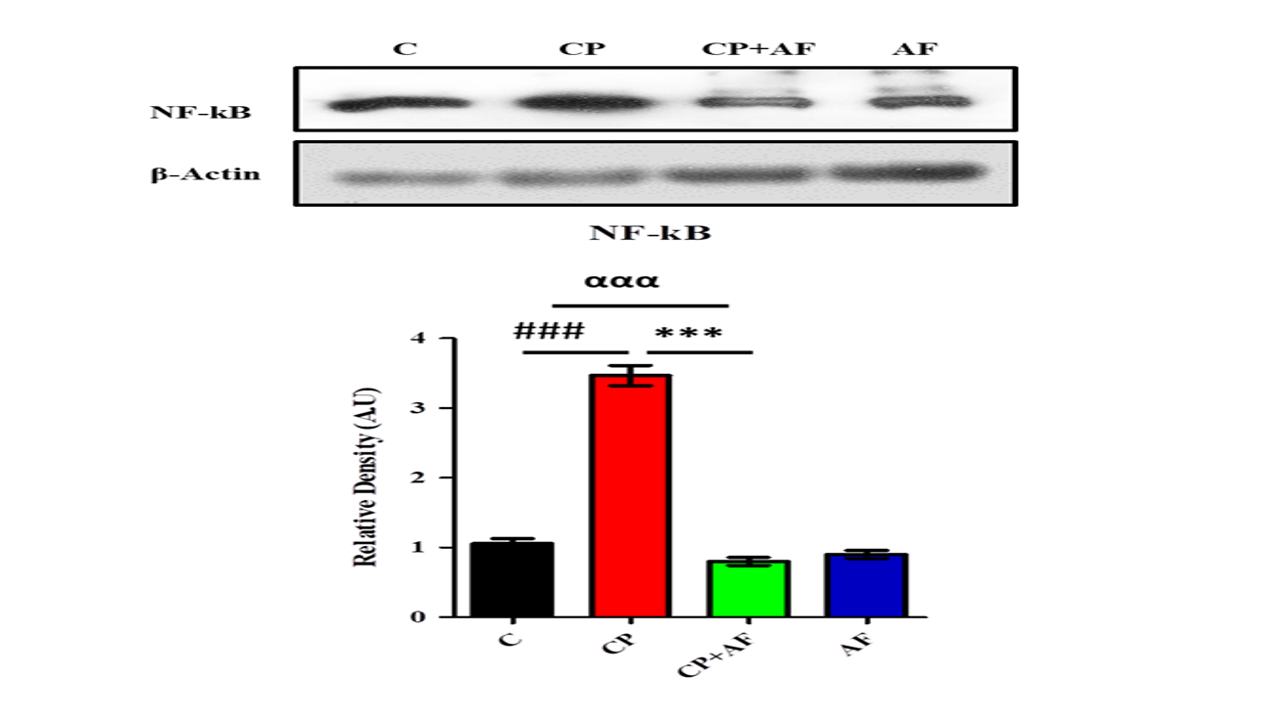
Figure 2: 6-AF demonstrated substantial amelioration in the expression level of TNF-α protein in the PND-7 mice brains of the cisplatin +6-AF group (shown are the Western blot results of TNF-α expression level, along with their bar-chart representation in various experimental mouse groups, respectively). β-Actin was used as a loading control. The results were determined using Image J software, and the bar chart indicates the mean in A.U. ± SEM. The significance of one-way ANOVA is expressed as α, the sign significance of control vs. cisplatin is expressed as #, and the significance of cisplatin vs. cisplatin +6-AF is expressed as*. Significance: (***, ###p<0.001).

Figure 3: 6-AF exhibited significant retrieval in the expression level of NFκB protein in the PND-7 mice brains of the cisplatin +6-AF group (shown are the Western blot results of NFκB expression level, along with their bar-chart representation in variousexperimental mouse groups, respectively). β-Actin was used as a loading control. The results were determined using Image J software, and the bar chart indicates the mean in A.U. ± SEM. The significance of a one-way ANOVA is expressed as α, the significance of a one-way ANOVA is expressed as α, the significance of control vs. cisplatin is expressed as #, and the significance of cisplatin vs. cisplatin +6-AF is expressed as*. Significance: (***, ###p<0.001).
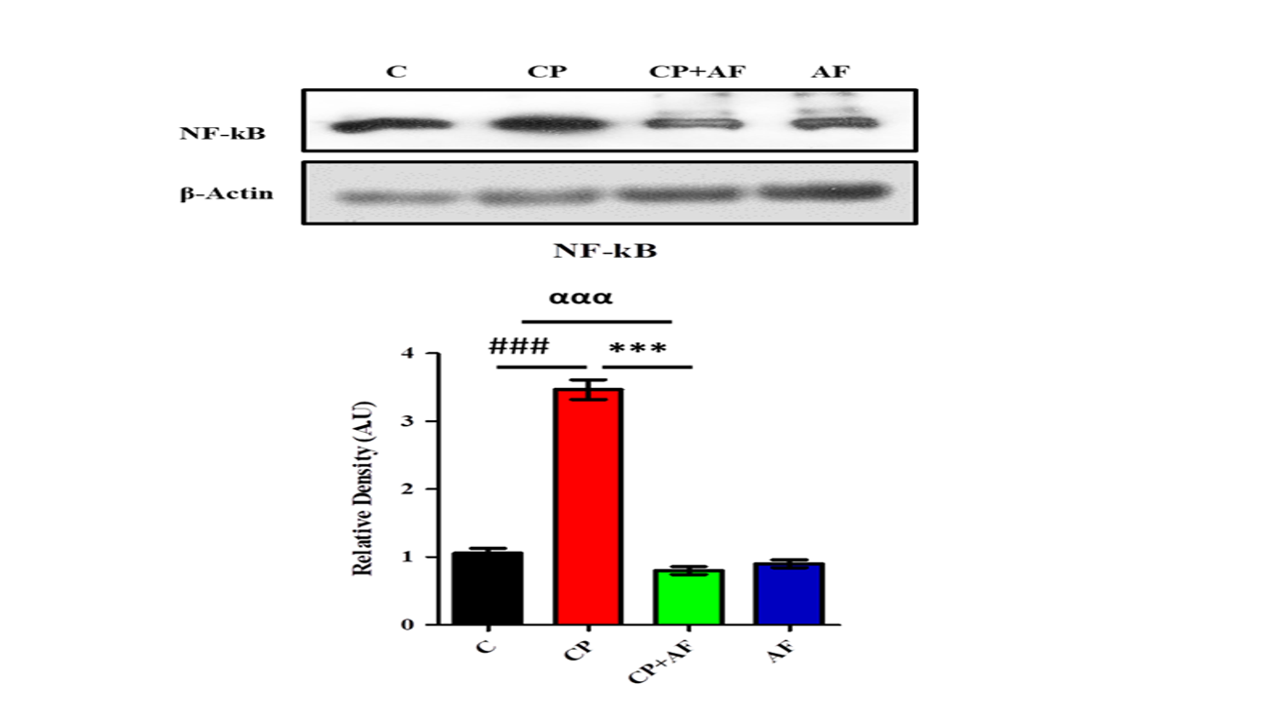
Figure 4: 6-AF successfully rectified the expression level of IL-1β protein in the PND-7 mice brains of the cisplatin +6-AF group (shown are the Western blot results of IL-1β expression level, along with their bar-chart representation in various experimental mouse groups, respectively). β-Actin was used as a loading control. The results were determined using Image J software, and the bar chart indicates the mean in A.U. ± SEM. The significance of one-way ANOVA is expressed as α, the significance of control vs. cisplatin is expressed as #, and the significance of cisplatin vs. cisplatin +6-AF is expressed as*. Significance: (***, ###p<0.001).
DISCUSSION
Our study demonstrated that 6-AF effectively mitigates cisplatin-induced neuro-inflammation in PND-7 mice. Analyses of brain homogenates revealed a significant increase (p<0.001) in p-JNK, COX-2, NF-κB, and IL-1β in cisplatin-treated mice compared to the control group. Conversely, cisplatin + 6-AF treatment resulted in a significant decrease (p<0.001) in the expression of these markers compared to the cisplatin-only group.
An increased inflammatory response has been associated with the development of cisplatin-induced neuro-inflammation.13 Many studies have reported that pro-inflammatory cytokine induction contributes to the pathogenesis and exacerbation of nervous tissue damage in cisplatin administered animals.14 To reduce inflammation in cisplatin-induced neurotoxicity, several in vivo approaches have been recommended, but it is unclear whether such approaches limit the anticancer efficacy of cisplatin.15
C-Jun N-terminal protein kinase (JNK) belongs to mitogen activated protein kinase (MAPK) superfamily.16 JNK can specifically phosphorylate the transcription factor c-Jun on its N-terminal transactivation domain at two serine residues, Ser63 and Ser73.17 Subsequent studies revealed that JNK also phosphorylates and regulates the activity of transcription factors and non-transcription factors, such as neuroinflammatory and neurodegenerative factors, in response to a variety of extracellular stimuli.18,19
Inflammatory disease therapies specifically target the JNK pathway. The synthesis of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α, along with the maturation and activity of T cells, are regulated by JNK.20 Several recent studies have established the importance of the JNK pathway in chronic inflammatory disorders involving the expression of specific proteases and cytokines.21 Depending on the stimulus and cell type, JNKs can phosphorylate several members of neuro-inflammation and apoptosis-related proteins.22
Multiple studies have demonstrated that pro-inflammatory cytokine induction, together with macrophage and neutrophil infiltration, contributes to the pathogenesis and exacerbation of many diseases in cisplatin-administered animals.13,23 Hence, inflammatory reaction modulation may help to prevent cisplatin-induced neuro-inflammation. Although different treatment strategies have been proposed to decrease cisplatin-induced inflammation, it is not clear whether such treatment strategies alter the anticancer efficacy of cisplatin.24
Several cytokines (e.g., TNF-α, IL-1β, MCP-1, and IL-6) are upregulated in the inflammatory cascade triggered by cisplatin.25 TNF-α plays a key role in cisplatin-induced neuro-inflammation by activating a variety of signaling pathways with cellular responses ranging from the activation of inflammation to cell death.26 Strong evidence affirm the vital role of NF-κB activation in the pathogenesis of cisplatin-induced neuro-inflammation.27 NF-κB regulates neutrophil, macrophage, and lymphocyte biology, as well as the synthesis of hundreds of proinflammatory genes, including TNF-α, thus amplifying the process of inflammation.28
A study reported that the liver and kidney contents of the inflammatory mediators TNF-α and NF-κB are increased along with the infiltration of neutrophils by cisplatin.29 The crucial pro-inflammatory mediator TNF-α is released by macrophages activated by neutrophils. TNF-α, then, by binding to its receptor, promotes the release of NF-κB.30 Furthermore, the subsequent release of NF-κB and its translocation into the nucleus activate the transcription of multiple genes, including IL-1β and TNF-α.31 These findings are very consistent with our findings. Our study revealed that cisplatin administration induced increased activation of the p-JNK protein along with the significant increase in its downstream inflammatory mediators TNF-α, NFκB and IL-1β.
Flavonoids are phenolic compounds that are currently under major attention in the field of drug discovery. Researchers used kaempferol, a naturally occurring flavonoid, against cisplatin-induced nephrotoxicity by modulating oxidative stress, inflammation, and apoptosis via the ERK and NF-κB pathways. They observed that, Kaempferol pre-treatment decreased the release of pro-inflammatory cytokines (IL-12 and TNF-α) and also modulated the levels of NF-κB, thereby ameliorating the cisplatin mediated inflammation.32 Lu L et al., (2023) utilized the flavonoid derivative DMXAA to mitigate cisplatin-induced acute kidney injury.33 Altindağ F et al., (2022) employed silymarin to address cisplatin-induced nephrotoxicity in Albino Wistar male rats, revealing reduced TNF-α and NF-kB expressions and increased IL-10 expression with silymarin treatment.34 Alsayari et al., (2019) demonstrated that Aurone, a benzofuranone, targets various mechanisms in cancer cells because of many possible targets, including cyclin-dependent kinase, telomerase, histone deacetylase, sirtuins, adenosine receptor, and microtubules.35 Fisetin, another flavonoid, exhibits antioxidant and anti-inflammatory effects,36 while flavonols from cinnamon activate Nrf2, combating oxidative stress.37
In this study, we demonstrated for the first time that 6-AF mitigates cisplatin-induced neuro-inflammation in PND 7 mice brains by inhibiting p-JNK activation and reducing key pro-inflammatory cytokines such as TNF-α, NF-kB, and IL-1. Our findings align with previous research, showing a significant increase in these inflammatory markers in cisplatin-treated PND 7 mice brains. Treatment with 6-AF notably decreased these proinflammatory markers, thus alleviating inflammatory damage. The mechanism underlying this protection—whether causal or consequential—remains to be clarified. Our results suggest that 6-AF has potential as an adjunctive therapy in cancer treatment, offering protection against cisplatin-induced neuro-inflammation.
CONCLUSION
In conclusion, 6-amino flavone decreased cisplatin-induced neurotoxicity in the PND-7 mice brain. The neuroprotective effect observed was associated with suppression of p-JNK protein along with its downstream TNF-α, NF-κB, and IL-1β proteins in the brain homogenates of PND 7 mice. Hence, we got strong evidence from a recent study that 6-amino flavone has higher efficacy against cisplatin-induced neuro-inflammation.
RECOMMENDATION
Future studies are also needed to explore impact of different dose of these drugs (6-amino flavone) by which above cisplatin induced neuro-inflammation can be reduced.
REFERENCES
1. Yan D, An G, Kuo MT. C-Jun N-terminal kinase signalling pathway in response to cisplatin. J Cell Mol Med 2016;20(11):2013-19. https://doi.org/10.1111/jcmm.12908
2. Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020;27(1):1-30. https://doi.org/10.1186/s12929-019-0592-z
3. Aldossary SA. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharmacol J 2019;12(1):7-15. https://dx.doi.org/10.13005/bpj/1608
4. Feliu J, Heredia-Soto V, Gironés R, Jiménez-Munarriz B, Saldaña J, Guillén-Ponce C, et al. Management of the toxicity of chemotherapy and targeted therapies in elderly cancer patients. Clin Transl Oncol 2020;22:457-67. https://doi.org/10.1007/s12094-019-02167-y
5. Gwathmey KG. Sensory neuronopathies. Muscle Nerve 2016;53(1):8-19. https://doi.org/10.1002/mus.24943
6. Cankara FN, Günaydın C, Çelik ZB, Şahin Y, Pekgöz Ş, Erzurumlu Y, et al. Agomelatine confers neuroprotection against cisplatin-induced hippocampal neurotoxicity. Metab Brain Dis 2021;36(2):339-49. https://doi.org/10.1007/s11011-020-00634-y
7. Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer 2021;21(1):37-50. https://doi.org/10.1038/s41568-020-00308-y
8. Romani AMP. Cisplatin in cancer treatment. Biochem Pharmacol 2022;206:115323. https://doi.org/10.1016/j.bcp.2022.115323
9. Guven H, Arici A, Simsek O. Flavonoids in our foods: A short review. J Basic Clin Health Sci 2019;3(2):96-106. https://doi.org/10.30621/jbachs.2019.555
10. Patel DK. Pharmacological activities and therapeutic potential of kaempferitrin in medicine for the treatment of human disorders: A review of medicinal importance and health benefits. Cardiovasc Haematol Disord Drug Targets 2021;21(2):104-14. https://doi.org/10.2174/1871529X21666210812111931
11. Souiei S, Bouajila J, Saidi I, Znati M, Jannet HB, El Garah F. Synthesis, in vitro and in silico evaluation of new flavonoids-trifluoroacetylated amino acid conjugates as anti-acetylcholinesterase and anti-proliferative agents. J Mol Struct 2023;1292:136180. https://doi.org/10.1016/j.molstruc.2023.136180
12. Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO. Melatonin Stimulates the SIRT 1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther 2017;23(1):33-44. https://doi.org/10.1111/cns.12588
13. Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci 2019;26(1):1-14. https://doi.org/10.1186/s12929-019-0518-9
14. Jayaram, S, Krishnamurthy PT. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of parkinson’s disease: The therapeutic role of Nrf2 activators. Neurochem Int 2021;145:105014. https://doi.org/10.1016/j.neuint.2021.105014
15. Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol 2019;32(8):1469-86. https://doi.org/10.1021/acs.chemrestox.9b00204
16. Cao M, Liu F, Ji F, Liang J, Liu L, Wu Q, et al. Effect of C-Jun N-terminal kinase (JNK)/P38 mitogen-activated protein kinase (P38 MAPK) in morphine-induced tau protein hyperphosphorylation. Behav Brain Res 2013;237:249-55. https://doi.org/10.1016/j.bbr.2012.09.040
17. Xie X, Kaoud TS, Edupuganti R, Zhang T, Kogawa T, Zhao Y, et al. C-Jun N-terminal kinase promotes stem cell phenotype in triple-negative breast cancer through upregulation of notch1 via activation of C-Jun. Oncogene 2017;36(18):2599-608. https://doi.org/10.1038/onc.2016.417
18. Nailwal NP, Doshi GM. Role of intracellular signaling pathways and their inhibitors in the treatment of inflammation. Inflammopharmacology 2021;29(3):617-40. https://doi.org/10.1007/s10787-021-00813-y
19. Sharma VK, Singh TG, Singh S, Garg N, Dhiman S. Apoptotic pathways and alzheimer’s disease: Probing therapeutic potential. Neurochem Res 2021;46:3103-22. https://doi.org/10.1007/s11064-021-03418-7
20. Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev 2018;44:38-50. https://doi.org/10.1016/j.cytogfr.2018.10.002
21. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018;9(6):7204-18. https://doi.org/10.18632/oncotarget.23208
22. Sun W, Zhang N, Liu B, Yang J, Loers G, Siebert HC, et al. HDAC3 inhibitor RGFP966 ameliorated neuroinflammation in the cuprizone-induced demyelinating mouse model and LPS-stimulated BV2 cells by downregulating the P2X7R/STAT3/NF-κB65/NLRP3 activation. ACS Chem Neurosci 2022;13(17):2579-98. https://doi.org/10.1021/acschemneuro.1c00826
23. Jha AK, Gairola S, Kundu S, Doye P, Syed AM, Ram C, et al. Toll-like receptor 4: An attractive therapeutic target for acute kidney injury. Life Sci 2021;271:119155. https://doi.org/10.1016/j.lfs.2021.119155
24. Fang C, Lou D, Zhou L, Wang J, Yang B, He Q, et al. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin 2021;42(12):1951-69. https://doi.org/10.1038/s41401-021-00620-9
25. Suzdaltseva Y, Goryunov K, Silina E, Manturova N, Stupin V, Kiselev SL. Equilibrium among inflammatory factors determines human MSC-mediated immunosuppressive effect. Cells 2022;11(7):1210. https://doi.org/10.3390/cells11071210
26. Saral S, Topçu A, Alkanat M, Mercantepe T, Akyıldız K, Yıldız L, et al. Apelin-13 activates the hippocampal BDNF/TrkB signaling pathway and suppresses neuroinflammation in male rats with cisplatin-induced cognitive dysfunction. Behav Brain Res 2021;408:113290. https://doi.org/10.1016/j.bbr.2021.113290
27. Wen L, Tao S, Guo F, Li L, Yang H, Liang Y, et al. Selective EZH2 inhibitor Zld1039 alleviates inflammation in cisplatin-induced acute kidney injury partially by enhancing RKIP and suppressing NF-κB P65 pathway. Acta Pharmacol Sin 2022;43(8):2067-80. https://doi.org/10.1038/s41401-021-00837-8
28. Afshari AR, Sanati M, Mollazadeh H, Kesharwani P, Johnston TP, Sahebkar A. Nanoparticle-based drug delivery systems in cancer: A focus on inflammatory pathways. Semin Cancer Biol 2022;86:860-72. https://doi.org/10.1016/j.semcancer.2022.01.008
29. Qin X, Meghana K, Sowjanya NL, Sushma KR, Krishna CG, Manasa J, et al. Embelin attenuates cisplatin-induced nephrotoxicity: Involving inhibition of oxidative stress and inflammation in addition with activation of Nrf-2/Ho-1 pathway. Biofactors 2019;45(3):471-8. https://doi.org/10.1002/biof.1502
30. Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev 2018;44:38-50. https://doi.org/10.1016/j.cytogfr.2018.10.002
31. Lei CQ, Wu X, Zhong X, Jiang L, Zhong B, Shu HB. USP19 inhibits TNF-α–and IL-1β–triggered NF-κB activation by deubiquitinating TAK1. J Immunol 2019;203(1):259-68. https://doi.org/10.4049/jimmunol.1900083
32. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63(3):364-74. https://doi.org/10.1007/s11427-020-1643-8
33. Lu L, Liu W, Li S, Bai M, Zhou Y, Jiang Z, et al. Flavonoid derivative DMXAA attenuates cisplatin-induced acute kidney injury independent of STING signaling. Clin Sci (Lond) 2023;137(6):435-52. https://doi.org/10.1042/CS20220728
34. Altindağ F. Silymarin ameliorates cisplatin-induced nephrotoxicity by downregulating TNF-α and NF-kB and by upregulating IL-10. J Exp Clin Med 2022;39(1):216-20. https://doi.org/10.52142/omujecm.39.1.42
35. Alsayari A, Muhsinah AB, Hassan MZ, Ahsan MJ, Alshehri JA, Begum N. Aurone: A biologically attractive scaffold as anticancer agent. Eur J Med Chem 2019;166:417-31. https://doi.org/10.1016/j.ejmech.2019.01.078
36. Naeimi AF, Alizadeh M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci Technol 2017;70:34-44. https://doi.org/10.1016/j.tifs.2017.10.003
37. Li AL, Li GH, Li YR, Wu XY, Ren DM, Lou HX, et al. Lignan and flavonoid support the prevention of cinnamon against oxidative stress related diseases. Phytomedicine 2019;53:143-53. https://doi.org/10.1016/j.phymed.2018.09.022
Following author have made substantial contributions to the manuscript as under:
LKA & AJ: Study design, acquisition, analysis and interpretation of data, drafting the manuscript, approval of the final version to be published
RJ & SAS: Concept and study design, analysis and interpretation of data, critical review, approval of the final version to be published
Author agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work.
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |