![]() https://doi.org/10.35845/kmuj.2024.23417 CASE
REPORT
https://doi.org/10.35845/kmuj.2024.23417 CASE
REPORT
When B12 therapy fails: two case reports of intravenous vitamin B12 resistance in pernicious anemia
Zeeshan ![]() 1, Ali
Nawaz Khan 1, Salman Amer Sheikh 2, Syed Onaiz Anwar 3,
Saeed Khan Akhtar 3, Rafia Alvi 2
1, Ali
Nawaz Khan 1, Salman Amer Sheikh 2, Syed Onaiz Anwar 3,
Saeed Khan Akhtar 3, Rafia Alvi 2
|
1: Department of
Cardiology, PNS Shifa, Karachi, Pakistan
Email
Contact #: +92-333- 7354779
Date Submitted: July 21, 2023 Date Revised: June 19, 2024 Date Accepted: July 03, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Zeeshan, Khan AN, Shiekh SA, Anwar SO, Akhtar SK, Alvi R. When B12 therapy fails: two case reports of intravenous vitamin B12 resistance in pernicious anemia. Khyber Med Univ J 2024;16(3):266-9. https://doi.org/10.35845/kmuj.2024.23417 |
ABSTRACT
BACKGROUND: Pernicious anemia is an autoimmune disorder causing vitamin B12 deficiency resulting from impaired absorption caused by intrinsic factor absence. Positive parietal cell and intrinsic factor antibodies confirm the diagnosis. Intravenous vitamin B12 effectively treats Pernicious anemia. Severe cytopenias (anemia, thrombocytopenia, neutropenia) may result, sometimes requiring emergency care and hospitalization.
CASE PRESENTATION: Case A: A 54-year-old male presented with six months of generalized fatigue and dyspnea on exertion. Laboratory tests revealed severe macrocytic anemia (Hb: 4.7 g/dL, MCV: 120 fL), with a peripheral blood smear showing hyper-segmented neutrophils and macro-ovalocytes, suggestive of megaloblastic anemia. Bone marrow biopsy demonstrated decreased cellularity with megaloblastic changes. Despite six-weeks of intravenous vitamin B12 therapy, there was no improvement in hematological parameters. Endoscopy revealed atrophic gastritis, and biopsy showed anti-parietal cell antibodies. Following a trial of immunosuppressive therapy with cyclosporine, no significant response was observed. Bone marrow transplantation was recommended.
Case B: A 62-year-old male presented with epigastric pain, dyspepsia, fatigue, and a three-month history of anorexia. He was diagnosed with macrocytic anemia (Hb: 6.7 g/dL, MCV: 100 fL), and peripheral blood smear indicated megaloblastic changes. Bone marrow biopsy confirmed decreased cellularity and megaloblastic anemia. Despite standard intravenous vitamin B12 therapy, the patient showed no hematological improvement. Endoscopic findings revealed atrophic gastritis. Immunosuppressive therapy with cyclosporine also failed, leading to the recommendation of bone marrow transplantation.
CONCLUSION: Continued efforts in understanding the pathophysiology of Pernicious anemia and autoimmune-mediated bone marrow failure syndromes may pave the way for innovative therapeutic approaches in the future.
KEYWORDS: Anemia (MeSH); Pernicious Anemia (MeSH); Megaloblastic Anemia (MeSH); Vitamin B 12 (MeSH); B-12 Therapy (Non-MeSH); Autoimmune Diseases (MeSH); Autoimmune disorder (Non-MeSH); Intrinsic Factor (MeSH); Cytopenia (MeSH).
INTRODUCTION
Pernicious anemia is a type of megaloblastic anemia caused by the deficiency of vitamin B12, typically due to impaired absorption resulting from the autoimmune destruction of gastric parietal cells.1 Although prompt administration of IV vitamin B12 supplementation is the standard treatment as it bypasses the gastrointestinal tract, 2 a subset of patients may fail to respond adequately, necessitating further investigation and alternative treatment options. 3
CASE PRESENTATION
Case A: A 54-year-old male patient reported experiencing generalized fatigue, increasing over the past six months. He also described dyspnea on exertion, limiting his physical activities. Initial laboratory investigations demonstrated severe macrocytic anemia, with a hemoglobin level of 4.7g/dL (reference range: 13.5-17.5 g/dL), MCV at presentation was 120fL after blood transfusion of 1-pint RCC MCV was 112fL and lowered to 98fL in week 3 and 4 of admission (reference range MCV: 80-100fL). All other investigations are mentioned in Table I. Peripheral blood smear examination revealed hyper-segmented neutrophils and macro-ovalocytes, consistent with megaloblastic changes. Bone marrow biopsy done revealed decreased cellularity in all hematopoietic lineages along with megaloblastic changes.
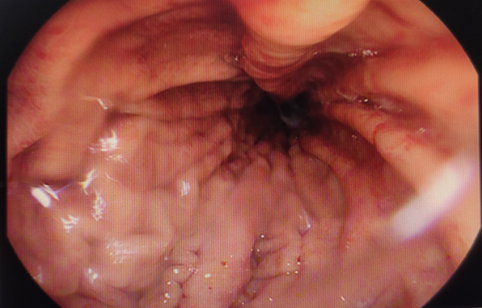
Figure 1: Case A: The stomach on endoscopy shows decrease mucosal folds with mucosal erythema and erosions throughout the stomach.
Case B: A 62-year-old male presented to the gastroenterology clinic with complaints of severe epigastric burning pain, dyspepsia, loss of appetite, and fatigue for three months. He reported experiencing significant discomfort after meals, which further contributed to his decreased appetite.
Figure 2: Case A: Anti-parietal cell antibodies of PCA
(LKS) pattern seen on endoscopic biopsy from the stomach, associated with
Type-A Atrophic Gastritis. Routine
blood tests revealed a low hemoglobin level of 6.7g/dL (reference range:
13.5-17.5 g/dL) and MCV 100fL at presentation, after transfusion with 1-pint
RCC MCV was 96.1fL (reference range MCV: 80-100fL). Other laboratory parameters
as mentioned in Table II. Peripheral blood smear examination revealed
hyper-segmented neutrophils and macro-ovalocytes, supporting the diagnosis of
megaloblastic anemia. Due to minimal response to Intravenous Vit B12 therapy,
bone marrow biopsy was advised which revealed decreased cellularity in all
hematopoietic lineages along with megaloblastic changes consistent with MDS. Diagnosis
and treatment: Both patients were admitted
for intravenous (IV) vitamin B12 therapy due to suspected pernicious anemia.
Intravenous (IV) vitamin B12 therapy was initiated following the standard
treatment protocol. 4,5 In both patients 1000µg IV once daily for 7
days. After that 1000µg IV was administered once weekly for 5 weeks during
their hospital stay. Serum potassium levels of both patients were measured
during and after administration which were within normal limits. No
hypersensitivity reactions were observed in either of the patients. However
on follow up, despite six weeks of regular treatment, there was no improvement
in the patient's hematological parameters. Despite the ongoing treatment and close monitoring of
complete blood counts (CBC), both patients failed to respond adequately. Table I
and II show lab parameters of case A and B done throughout their hospital stay.
Endoscopic examinations revealed atrophic gastritis in both cases. Figure 2
shows anti-parietal cell antibodies seen on endoscopic biopsy from the stomach
in case A, associated with type A atrophic gastritis. The bone marrow biopsies
of both patients revealed hypocellularity, with reduced cellularity in all hematopoietic lineages. Megaloblastic changes
were evident, characterized by dysplastic features in erythroid and myeloid
precursors. Importantly, no lymphocyte or plasma cell infiltrates were
observed, ruling out lymphoproliferative disorders as a cause of hypocellularity.
The lack of response to vitamin B12 therapy and positive serological tests, a
trial of immunosuppressive therapy was given. The patients were administered
cyclosporine at a dose of 5 mg/kg/day for 4 weeks. 6 However, there
was no significant improvement in the hematological parameters. Considering
the failure of conventional therapies, a multidisciplinary team discussion
recommended the patients for bone marrow transplantation. The patients are
planned to undergo extensive evaluation for eligibility, including tissue
typing, cardiopulmonary assessment, and infectious disease screening. Table I:
Case A summary of laboratory results from admission to discharge Blood Counts Week 1 Post Transfusion Week 2 Week 3 Reference Values TLC
(109/L) 3.8 5.2 5.2 5.3 4-10 Hb
(g/dl) 4.7 6.4 7.9 8.6 13.5-17.5 HCT
(%) 15.1 18 25.1 24.5 40-52 MCV
(fl) 120 112 98 98.4 80-100 Platelets
(109/L) 17 38 21 16 150-400 Serum
Folate (ηg/mL) 3.55 3-12 Vitamin
B-12 (ρmol/L) >1476 138-652 Retic
Count (%) 3.45 0.3 0.5-1.5 TLC: Total leukocyte count; Hb: Hemoglobin; HCT:
Hematocrit; MCV: Mean corpuscular volume. Table
II: Case B summary of laboratory results from admission till discharge Blood Counts Week 1 Post Transfusion Week 2 Week 3 Reference values TLC
(109/L) 2.1 3 3.3 3.6 4-10 Hb
(g/dl) 6.7 9.7 9.2 8.1 13.5-17.5 HCT
(%) 20.1 29.7 28.4 24.7 40-52 MCV
(fl) 100 96.1 97 97.2 80-100 Platelets
(109/L) 18 33 14 10 150-400 Serum
Folate (ηg/mL) 2.37 3-12 Vitamin
B-12 (ρmol/L) 111.9 138-652 Retic
Count (%) 1.03 0.4 0.5-1.5 TLC:
Total leukocyte count; Hb: Hemoglobin; HCT: Hematocrit; MCV: Mean corpuscular
volume. DISCUSSION Pernicious
anemia, a condition characterized by the inability to absorb vitamin B12 due to
intrinsic factor deficiency, is a common cause of vitamin B12 deficiency. The
standard treatment for pernicious anemia is intravenous (IV) vitamin B12
therapy, which typically results in significant improvement of symptoms and
hematological parameters. 7 However, in rare cases, pernicious
anemia may coexist with other underlying hematological disorders, complicating
the diagnostic and therapeutic approach. 8 In
these cases, the patient presented with classic symptoms of pernicious anemia,
including fatigue, weakness, and paresthesia. Laboratory investigations
confirmed the diagnosis, with findings of macrocytic anemia, and low serum
vitamin B12 levels. The patient was initiated on IV B12 therapy, following the
standard dosing regimen. However, despite several weeks of treatment, there was
no improvement in symptoms, and the patient's hemoglobin levels remained
persistently low. The
bone marrow biopsy revealed decreased cellularity in all hematopoietic lineages
along with megaloblastic changes, consistent with myelodysplastic syndrome
(MDS). 9 Myelodysplastic syndromes are a heterogeneous group of
hematological disorders characterized by ineffective hematopoiesis, leading to cytopenia’s
and an increased risk of progression to acute myeloid leukemia. 8,9 The
coexistence of pernicious anemia and MDS explained the refractory anemia and
the lack of response to vitamin B12 replacement therapy. It is important to note
that MDS can present with macrocytic anemia, mimicking the clinical features of
pernicious anemia. Therefore, in patients with pernicious anemia who do not
respond to treatment, a bone marrow biopsy should be considered to identify
potential concurrent hematological disorders, such as MDS. 9 At
present mainstay of treatment for myelodysplastic syndromes includes: ·
Supportive
care: Many patients require frequent blood transfusions due to persistently low
blood counts and for symptoms of anemia such as shortness of breath and
fatigue. Erythropoiesis stimulating agents may be given to increase the number
of mature red blood cells in the body and reduce symptoms of anemia. ·
Drug
therapy: This can include immunosuppressive drugs to weaken the immune system
which reduces the need for blood transfusions. Other drugs like lenalidomide,
azacitidine and decitabine may be used ·
Stem
cell transplantation: Hematopoietic
stem cell transplantation (HSCT) offers the potential for cure, but timing of
the procedure may be important and a limiting factor. Most patients with MDS
are elderly and only a few young patients will have a matched donor, the use of
bone marrow transplantation is limited. CONCLUSION We presented two cases of pernicious anemia where
patients did not respond to IV vitamin B12 therapy and showed hypocellularity
on bone marrow examination. Despite 6 weeks of IV B12 and 4 weeks of
cyclosporine immunosuppressive therapy, their hematological parameters remained
unchanged. Bone marrow biopsies revealed myelodysplastic syndrome, prompting
consideration of HSCT. These cases highlight the complexity
and challenges in managing pernicious anemia when standard treatment approaches
fail. REFERENCES 1. Esposito
G, Dottori L, Pivetta G, Ligato I, Dilaghi E, Lahner E. Pernicious anemia: the
hematological presentation of a multifaceted disorder caused by cobalamin
deficiency. Nutrients 2022;14(8):1672. https://doi.org/10.3390/nu14081672 2. Laisk
T, Lepamets M, Koel M, Abner E, Metspalu A, Nelis M, et al. Genome-wide
association study identifies five risk loci for pernicious anemia. Nat Commun
2021;12(1):3761. https://doi.org/10.1038/s41467-021-24051-6 3. Htut
TW, Thein KZ, Oo TH. Pernicious anemia: Pathophysiology and diagnostic
difficulties. J Evid Based Med 2021;14(2):161-9. https://doi.org/10.1111/jebm.12435 4. Green R.
Vitamin B 12 deficiency from the perspective of a practicing hematologist. Blood
2017;129(19):2603-11. https://doi.org/10.1182/blood-2016-10-569186 5. Devalia
V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of
cobalamin and folate disorders. Br J Haematol 2014;166(4):496-513. https://doi.org/10.1111/bjh.12959 6. Shah
SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical practice update on the
diagnosis and management of atrophic gastritis: expert review. Gastroenterology
2021;161(4):1325-32.e7. https://doi.org/10.1053/j.gastro.2021.06.078 7. Fenaux
P, Platzbecker U, Ades L. How we manage adults with myelodysplastic syndrome.
Br J Haematol 2020;189(6):1016-27. https://doi.org/10.1111/bjh.16206 8. Garcia-Manero
G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on
diagnosis, risk stratification and management. Am J Hematol
2020;95(11):1399-420. https://doi.org/10.1002/ajh.25950 9. Lee P, Yim R,
Yung Y, Chu HT, Yip PK, Gill H. Molecular targeted therapy and immunotherapy
for myelodysplastic syndrome. Int J Mol Sci 2021;22(19):10232. https://doi.org/10.3390/ijms221910232 CONFLICT
OF INTEREST Authors declared
no conflict of interest, whether financial or otherwise, that could influence
the integrity, objectivity, or validity of their research work. GRANT
SUPPORT AND FINANCIAL DISCLOSURE Authors declared
no specific grant for this research from any funding agency in the public,
commercial or non-profit sectors DATA
SHARING STATEMENT The data that support the findings of this study are
available from the corresponding author upon reasonable request KMUJ web
address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk
![]() This is an Open Access article distributed under the terms of
the Creative Commons Attribution 4.0 International License.
This is an Open Access article distributed under the terms of
the Creative Commons Attribution 4.0 International License.