![]() https://doi.org/10.35845/kmuj.2024.23098 ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2024.23098 ORIGINAL ARTICLE
Comparison of vacuum-assisted closure device versus bolster dressing for securing skin grafts: a randomized controlled trial
Saira
Ahmed Chhotani![]() 1,
Faisal Akhlaq Ali Khan 1, Sindhu Khan
1,
Faisal Akhlaq Ali Khan 1, Sindhu Khan![]() 1,
Rabeeaa Farrukh
1,
Rabeeaa Farrukh![]() 1,
Sadaf Gulzar 1, Erum Naz1
1,
Sadaf Gulzar 1, Erum Naz1
|
1: Department of Plastic and Reconstructive Surgery, Dr. Ruth K.M. Pfau Civil Hospital Karachi, Pakistan
Email
Contact #: +92-301-2822996
Date Submitted: September 10, 2022 Date Last Revised: March28, 2024 Date Accepted: May 27, 2024 |
|
THIS ARTICLE MAY BE CITED AS: Chhotani SA, Khan FAA, Khan S, Farrukh R, Gulzar F, Naz E. Comparison of vacuum-assisted closure device versus bolster dressing for securing skin grafts: a randomized controlled trial. Khyber Med Univ J 2024;16(2):85-90. https://doi.org/10.35845/kmuj.2024.23098 |
ABSTRACT
OBJECTIVES: To compare the percentage of graft take and short-term post-operative complications between using a vacuum-assisted closure (VAC) device and a traditional bolster dressing for securing split-thickness skin grafts (STSGs) in traumatic wounds.
METHODS: This single-blinded, randomized controlled trial was conducted at the Department of Plastic and Reconstructive Surgery, Civil Hospital, Karachi, Pakistan, from October 21, 2021, to June 20, 2022. Patients aged 18 to 60 with soft tissue injuries undergoing skin grafting were included. After STSGs, participants were randomly assigned to either the VAC group or the bolster dressing group using a sequentially numbered opaque sealed envelope protocol. On the fifth post-operative day, the dressings were removed under consultant supervision, and the percentage of skin graft take and post-operative complications were assessed. Data were analyzed using SPSS version 21.
RESULTS: There were no significant differences in baseline characteristics like age (p=0.082), body mass index (p=0.770), co morbidities (diabetes, p=0.583; hypertension, p=0.237), graft site (p=0.583), and graft size (p=0.09) between the groups. Post-operative complications, including hematoma (8.6%), seroma (14.3%), and infection (5.7%), were more common in the bolster group compared to the VAC group. A statistically significant difference in seroma formation was observed between the two groups (p=0.001). Additionally, the proportion of graft take was significantly higher in the VAC group than in the bolster dressing group (94.3±4.2% vs. 85.6±4.4%, p=0.001).
CONCLUSION: The use of the VAC device resulted in fewer post-operative complications and a significantly higher percentage of successful graft take compared to the conventional Bolster dressing method.
Clinical Trial Registration Number: NCT05831020
KEYWORDS: Split Thickness Graft (MeSH),Graft-take (Non-MeSH), Vacuum-assistedclosure (MeSH), Seroma (MeSH), Bolster dressing (Non-MeSH), Soft Tissue Injuries (MeSH), Karachi (Non-MeSH), Pakistan (MeSH).
INTRODUCTION
Split-thickness skin grafting (STSG) is a widely used procedure to address both minor and significant skin defects.1 This technique involves transferring cutaneous tissue from a designated donor site to the targeted graft site. Although STSG is crucial for wound closure, improper pressure on the skin graft can result in infection, hemorrhage, dislocation, or graft loss, significantly reducing graft uptake. Thus, effective interventions are essential for graft survival and integration, which in turn, significantly influence wound healing and overall patient recovery.2,3
Over recent decades, various methods and dressings have been developed to stabilize and enhance the attachment of skin grafts to wound sites. These include adhesive dressings, pull-out tie-over dressings, sponge bolsters, and negative pressure dressings.2-5 Among these techniques, the vacuum-assisted closure (VAC) device, also known as negative pressure wound therapy (NPWT), and traditional bolster dressing have emerged as two prominent methods for securing skin grafts.3,4
The VAC device has gained popularity due to its potential to improve wound healing through the application of controlled negative pressure. This method reduces edema, promotes tissue perfusion, and encourages cellular proliferation.6 In contrast, traditional bolster dressings are commonly used for securing skin grafts, especially in cases of minor to moderate wounds. However, evidence suggests that conventional bolster dressings may not be optimal for exuding wounds, poorly healed regions, contoured recipient sites, or areas subjected to shear stress.7 these factors can significantly reduce the success of STSG, leading to increased pain, extended hospital stays, higher morbidity, and increased costs
While studies such as those by Mohsin M, et al.,9 provide valuable insights into the effectiveness of VAC over traditional dressings, particularly in terms of lower complication rates and better graft uptake, these findings may not fully represent the Pakistani healthcare environment where resources are limited. Research by Mujahid AM, et al,.6 conducted in Pakistan also highlighted the superiority of VAC in graft take and complication rates; however, it addresses only a single and small pre-operative graft size.
Despite the prevalent use of bolster dressing in Pakistan, there is a significant lack of local data comparing it to VAC, a modern wound care technology known for enhancing wound healing and graft success. Therefore, this study was planned to bridge this gap by providing a detailed comparison of VAC and traditional bolster dressing. The findings are expected to serve as a valuable resource for healthcare professionals, facilitating evidence-based decision-making when selecting the most appropriate skin graft securing method across various clinical scenarios.
METHODS
This study was conducted at the Department of Plastic and Reconstructive Surgery, Dr. Ruth K.M. Pfau Civil Hospital, Karachi, Pakistan, from October 21, 2021, to June 20, 2022. It adhered to the Helsinki protocol, and ethical approval was obtained from the Institutional Review Board prior to commencement.
Trial design
This randomized controlled trial featured two parallel groups. A total of 140 patients were randomly assigned to either Group A (VAC group, n=70) or Group B (bolster dressing group, n=70).
Participants
All patients aged 18 to 60 years undergoing skin grafts at the Civil Hospital, with ASA grade 1 and traumatic wounds sized between 25 cm² and 225 cm² of more than 14 days' duration with healthy granulation tissue, were included. Patients showing signs of wound infection, those with wounds with poor blood supply, or those known to have allergies or sensitivities to acrylic adhesive were excluded from the study. Participants in this trial were enrolled using non-probability consecutive sampling. Figure 1 depicts the CONSORT diagram illustrating the enrollment process, allocation of participants to treatment groups, follow-up procedures, and the planned analysis approach for the study.
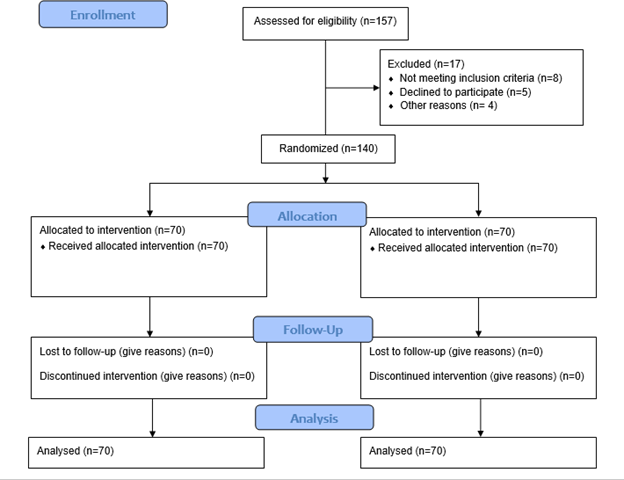
Figure 1: “CONSORT DIAGRAM”
Interventions
Skin grafting was performed under
general anesthesia. After debriding and washing the recipient site with normal
saline, a sterile scale was used to measure the wound size in two dimensions in
centimeters. A Humby's knife was used to harvest the skin graft from the donor
site, which was then manually meshed with a knife blade and securely attached
to the recipient site using 3-0 vicryl sutures. A layer of paraffin gauze
soaked in tincture of benzoin was applied over the graft.
In the bolster dressing group, a wet bulky cotton gauze dressing was applied to the recipient site, followed by wrapping with a cotton bandage dampened with 0.9% normal saline.
In the VAC group, sterilized VAC sponges were custom-fitted to match the wound's contour and positioned with a fenestrated tube inserted between the layers. This assembly was securely attached to the surrounding skin using tincture of benzoin spray and an adhesive dressing called OPSITE. The VAC was set to a suction of -125 mm Hg, and a thorough check was conducted to detect any potential air leaks. If a loss of suction was visualized by the absence of foam collapse and a gushing sound, the adhesive dressing was reinforced over the area of the air leak. The VAC remained decompressed and clamped until the patient was transferred back to the ward. Once in the ward, the VAC was attached to a wall-mounted suction apparatus (Danyang Excellent Medical Equipment Co., Ltd.) via another connecting rubber tube and set to -125mmHg10 suction intermittently for 2 hours, followed by decompression for the next 2 hours. The dressings were continually observed by the nursing staff and resident doctor for loss of suction. Depending on the injury's location, bed rest, a sling, or a splint was used for immobilization, while slight movement for personal hygiene was permitted. Patients underwent their first dressing change on the fifth post-operative day. However, if excessive dressing soakage with blood, serous fluid, foul-smelling discharge, or pus was present, an early dressing change was performed.
Outcomes
On the fifth post-operative day, the dressing of each patient was carefully removed by the researcher, supervised by an assistant professor and plastic surgery consultant. During this process, the percentage of skin graft take and any post-operative complications were thoroughly assessed. The negative pressure dressing was discontinued in Group A. Hematomas and seromas were drained using either a 10cc syringe or a small incision made with a no. 11 surgical blade. Infections were managed by sending a wound culture and sensitivity test (C/S) and using antibacterial dressings or administering oral and intravenous antibiotics, as needed. Thereafter, the patients were followed for an additional 2 weeks with alternate day dressings, or daily dressings in cases of infection.
We did not document graft take percentage or post-operative complications during follow-up dressings, as any further loss in graft take is typically attributed to patient non-compliance in restricting movement of the involved area or secondary infection due to unsterile dressings, rather than the initial method used to secure the STSG.
Sample size
The sample size of 140 (70 per group) was calculated using online Open Epi sample size calculator, by taking statistics of mean graft take for VAC group=96±6 and for conventionally treated group=89±203,level of confidence = 95% and power = 80%.
Randomization, allocation concealment, and blinding
Patients were randomly assigned to either the conventional group or the new technique group using the sequentially numbered opaque sealed envelope (SNOSE) protocol. A total of 140 sequentially numbered opaque sealed envelopes were prepared, with half labeled as Group A (VAC group) and the other half as Group B (bolster dressing group). The envelopes were then shuffled to ensure randomization. This was a single-blind study, with participants being blinded to their group assignment.
Data collection and management
Patients visiting the outpatient department and requiring skin grafts were admitted to the plastic surgery unit and recruited into the study with their consent. The study purpose, along with the associated risks and benefits, was explained to the patients to obtain informed consent. The date of admission was recorded, and demographic and clinical information was collected, including age, gender, socioeconomic status (low, middle, high), place of residence (rural, urban), presence of comorbid conditions (diabetes, hypertension), and smoking status. Height (in meters) and weight (in kilograms) were measured to calculate body mass index (BMI) [kg/m²]. The location of the defect was documented. Routine laboratory tests (complete blood count, viral markers, and wound culture) and radiological investigations (chest X-ray for anesthesia fitness) were performed before surgery. The duration of the wound (time from injury to surgery in days) and the date of surgery were also recorded.
Data storage
A pre-designed proforma was used to collect the data. Patients’ information was kept confidential by tagging medical record numbers with a separate serial number. Only the principal investigator had access to the original data, ensuring its confidentiality.
Data analysis
The data were analyzed using SPSS version 21. Categorical variables, including socioeconomic status, gender, place of residence, smoking status, medical comorbidities (such as diabetes mellitus and hypertension), graft site, donor site, and post-operative complications, were presented as frequencies with percentages. Quantitative variables such as age, height, weight, BMI, graft size, length of hospital stay, and percentage of graft take were summarized using mean and standard deviation. To compare graft take between two groups, an independent t-test or Mann-Whitney U test was employed as appropriate. Effect modifiers such as age, weight, height, socioeconomic status, gender, smoking and alcohol use, graft site, donor site, graft size, co morbidities, and post-operative complications were addressed through stratification. Following this, the independent t-test or Mann-Whitney U test was applied again. A significance level of 5% was considered statistically significant.
RESULTS
In our study, a total of 140 patients were enrolled, with 70 patients in each group. Among the participants, 78 (55.7%) were males and 62 (44.3%) were females. In the VAC group (Group A), there were 34 (48.6%) males and 36 (51.4%) females whereas in the bolster group (Group B), there were 44 (62.9%) males and 26 (37.1%) females.
The mean age was comparable between the two groups: 32.7±13.3 years in the VAC group and 36.43±11.6 years in the bolster group. Other baseline characteristics are displayed in Table I.
Table I: Baseline characteristics of both the study groups
|
Variables |
Group A (VAC group) (n=70) |
Group B (Bolster group) (n=70) |
p-value |
||
|
Mean |
SD |
Mean |
SD |
||
|
Age (years) |
32.71 |
13.38 |
36.43 |
11.62 |
0.082 |
|
Weight (kg) |
71.80 |
11.00 |
73.03 |
12.38 |
0.536 |
|
Height (cm) |
167.57 |
9.61 |
168.03 |
9.20 |
0.774 |
|
BMI (kg/m2) |
25.83 |
4.86 |
26.07 |
5.01 |
0.770 |
|
Graft size (cm2) |
95.98 |
23.61 |
98.32 |
21.35 |
0.09 |
BMI: Body mass index; Kg: kilogram; cm: centimeter; m: meter; VAC: vacuum assisted closure
The mean length of hospital stay was shorter in the VAC group (11.8±1.2 days) compared to the bolster group (14.3±2.16 days), with a p-value of 0.032. Socioeconomic status was similar in both groups (p-value 0.183), as was residential status (p-value 0.173). Smokers constituted 42.9% of the bolster group and 34.3% of the VAC group (p-value 0.298). Diabetes was present in 28.6% of patients in the bolster group and 32.9% in the VAC group (p-value 0.583). Hypertension was observed in 28.6% of the bolster group and 20% of the VAC group (p-value 0.237). Arm and forearm grafts were used in 28.6% of patients in the bolster group and 32.9% in the VAC group (p-value 0.583). The donor site was the contralateral thigh in 82.9% of patients in the bolster group and 77.1% in the VAC group (p-value 0.398). The proportion of graft take was higher in the VAC group compared to the bolster group (94.3±4.2 vs. 85.6±4.4, p-value 0.001).
Post-operative complications were more common in the bolster group, with hematoma occurring in 8.6% of patients, seroma in 14.3%, and infection in 5.7%. There was a statistically significant difference in seroma formation between the two groups (p=0.001), as shown in Table II.
Table II: Frequency of post-operative complications
|
Complications |
Group A (VAC group) (n=70) |
Group B (Bolster group) (n=70) |
p-value |
|
Hematoma |
2 (2.9%) |
6 (8.6%) |
0.106 |
|
Seroma |
0 (0%) |
10 (14.3%) |
0.001 |
|
Infection |
2 (2.9%) |
4 (5.7%) |
0.236 |
VAC: vacuum assisted closure
DISCUSSION
The management and coverage of complicated soft tissue wounds remain a challenge in patients who have suffered burns or traumatic injuries.11-13 Numerous methods are employed to achieve wound coverage, with one commonly used approach involving the placement of STSGs. The VAC is a modified dressing consisting of a sponge and suction tubing, secured to the wound with an occlusive dressing. The application of negative pressure through the suction tubing creates a continuous negative-pressure environment.3,14This device has shown promising results, including faster development of granulation tissue, earlier re-epithelialization of wounds, and accelerated healing of burn wounds. Moreover, it has been effectively utilized to manage highly complex wounds.3To contribute to the understanding of wound management,this study was conducted to compare the effectiveness of VAC with traditional bolster dressing for securing STSG.
In our study, a total of 140 patients were enrolled, with 70 patients in each group. The pre-operative graft size was comparable between the two groups, with 98.3±21.3 cm² in the Bolster group and 95.98±23.6 cm² in the VAC group. The mean length of hospital stay was shorter in the VAC group (11.8±1.2 days) compared to the Bolster group (14.3±2.16 days), with a p-value of 0.032. Post-operative complications were more common in the Bolster group, including hematoma (8.6%), seroma (14.3%), and infection (5.7%), with a p-value of 0.002. The proportion of graft take was higher in the VAC group compared to the Bolster group (94.3±4.2 vs. 85.6±4.4, p-value 0.001).
The results of our study are consistent with other studies. A study conducted in California found that the average graft take in the group treated with VAC was 96±6%, higher than the conventionally treated group, which had a mean graft take of 89±20%.3 Similarly, an Indian study observed that the mean graft take was better in the NPWT group, which was 99.74±0.73%,9 compared to the non-NPWT group, which had a mean graft take of 88.52±9.47%.Moreover, no major complications were observed with NPWT treatment.9 In contrast, Lee and Kim reported complications of hematoma and seroma in 8.5% and 1.7% of cases, respectively.15
In another study, sixty-one patients underwent STSG placement for various indications, including burn injuries (n = 32), soft tissue loss (n = 27), and fasciotomy-site coverage (n = 2). The patients were divided into two groups, with 34 patients treated using the VAC and 27 patients treated with the bolster dressing. The study revealed that the VAC group had a significantly lower need for repeated STSGs compared to the no-VAC group (3% vs. 19%, p=0.04). There were no significant differences in terms of age or hospital length of stay between the two groups. However, it was observed that the no-VAC group had significantly larger grafts in comparison to the VAC group (p=0.006). Notably, the VAC group did not experience any dressing-associated complications during the study.3 While our study yielded similar results, one distinction was that the length of hospital stay in the VAC group was shorter, which was not documented in the other study? This difference may be attributed to the early discharge policy at our hospital.
According to Waltzman JT,.16, the VAC has shown significant improvements in wound healing outcomes. They found in total, 88 skin graft sites were secured with a VAC. The average grafted area was 367 ± 545 cm, with the most common graft sites being the leg, thigh, and arm (28%, 15%, and 12% respectively). The average percent graft take was 99.5 ± 1.5%, and remarkably, no patients required repeat grafting in the operating room. The average time for complete re-epithelialization was 16 ± 7 days.16In comparison, our study's results were similar, except for the graft site, which was smaller in our group. This difference can be attributed to the variation in patient selection between the two studies, as Waltzman JT,.16 enrolled burn patients, while our study included soft tissue injury patients.
Petkar and colleagues conducted a study akin to ours, which was published in the European Journal of Plastic Surgery. Their research entailed the implementation of split skin grafting on 71 wounds, where 35 of the wounds were subject to a vacuum-closure assembly that was linked to a continuous wall-suction of 80 mm Hg for duration of 4 days (cases). The remaining 36 wounds were treated with a Bolster dressing (controls).17 The study included 64 patients, with 43 males and 29 females. The grafted wounds encompassed a variety of conditions, including traumatic wounds, fresh surgically created wounds, chronic and acute burn wounds, diabetic wounds and post-inflammatory wounds. The graft take was evaluated in both groups at 9th day and 2nd week, and duration of dressing were compared in both groups. The results showed that in the study group, the final graft take at two weeks ranged from 70% to 100%, with an average of 95.29% graft take (SD: 5.9). In contrast, the control group exhibited a graft take ranging between 0% to 100%, with an average graft take of 85.89% (SD: 25.1). The dressing duration for the grafts in the cases was 11.63 days, whereas in the controls, it was 15.11 days. These differences were statistically significant. It's worth noting that the methodology and outcomes were similar to our study, except that Petkar K, et al., used a continuous negative pressure of 80mmHg, while we utilized 125mmHg intermittently. Nevertheless, the results obtained in their study were comparable to ours. Moreover, our findings are supported by other similar studies.18-22
The study conducted by Mujahid AM, et al., in Pakistan, enrolled 120 patients with soft tissue defect over scalp, after trauma, burn or tumor excision. The mean defect size was 9.10 ±2.16 cm. The patients were divided into two groups, with 60 patients treated with simple dressing after STSG (Group A) and 60 patients treated with VAC (Group B). The graft-take with VAC dressing was better than simple dressing (93.3% vs 40%, p-value 0.0001). The VAC group also had lower complication rate, i.e. seroma (1.67% vs 13.3%, p= 0.015) and hematoma (0.0% vs 6.67%, p=0.042).The results were comparable with our study; however, few distinctions are notable. The study did not include patients with co-morbidities (hypertension and diabetes mellitus), while ours did. Also Mujahid et al6 used continuous negative pressure at 50mmHg for 3 days, while we applied intermittent negative pressure at 125mmHg for 4 days. The study conducted by Mujahid et al6 only included one graft site i.e. scalp, which is attributable to a much smaller graft size (9.10±2.16 cm). The present study includes a wider range of graft site selection (upper and lower limb) with pre-operative graft size (97.14±22.45 cm2).
Although the current study was a randomized controlled trial, there were a few limitations in our study. The study did not include fresh surgical wounds or burn wounds, limiting the wound size dimension to less than 225 cm². Additionally, patient satisfaction was not reported, which could provide valuable insights for reconstructive surgeons in our local settings and contribute to improved practice. We propose a future study to address these limitations and further enhance our understanding and application of wound management techniques.
CONCLUSION
Vacuum-assisted closure is a better option than bolster dressing in terms of increased graft take and reduced complication rates. Additionally, the length of hospital stay is shorter in the VAC group compared to the bolster dressing group, which decreases the financial burden and allows for an earlier return to daily activities.
REFERENCES
1. Braza ME, Fahrenkopf MP. Split-Thickness Skin Grafts. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2023.[ Accessed on: January 25, 2024]. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK551561/.
2. Chou PR, Wu SH, Hsieh MC, Huang SH. Retrospective study on the clinical superiority of the vacuum-assisted closure system with a silicon-based dressing over the conventional tie-over bolster technique in skin graft fixation. Medicina (Kaunas) 2019;55(12):781.. https://doi.org/10.3390/medicina55120781
3. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137(8):930-3; discussion 933-4. https://doi.org/10.1001/archsurg.137.8.930.
4. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Prob Surg 2014;51(7):301-31. https://doi.org/10.1067/j.cpsurg.2014.04.001.
5. Steele L, Brown A, Xie F. Full-thickness skin graft fixation techniques: a review of the literature. J Cutan Aesthet Surg 2020;13(3):191-6. https://doi.org/10.4103/jcas.Jcas_184_19.
6. Mujahid AM, Khalid FA, Ali N, Sajjad Y, Khan H, Tarar MN. Vacuum-assisted closure in integration of skin graft over scalp wounds: a randomised control trial. J Coll Physicians Surg Pak 2020;30(2):163-7. https://doi.org/10.29271/jcpsp.2020.02.163.
7. Buller M, Lee TJ, Davis J, Wilhelmi BJ. Bolstering skin grafts with a surgical scrub brush: a cost-effective solution. Eplasty 2017;17:e21.
8. Azzopardi E, Boyce D, Dickson W, Azzopardi E, Laing H, Whitaker I, shokrollahi K. Application of topical negative pressure (vacuum-assisted closure) to split-thickness skin grafts a structured evidence-based review. Ann Plast Surg 2013;70:23-9. https://doi.org/10.1097/SAP.0b013e31826eab9e
9. Mohsin M, Zargar HR, Wani AH, Zaroo MI, Baba PUF, Bashir SA, et al. Role of customised negative-pressure wound therapy in the integration of split-thickness skin grafts: A randomised control study. Indian J Plast Surg 2017;50(1):43-9. https://doi.org/10.4103/ijps.IJPS_196_16
10. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma 2019;10(5):845-8. https://doi.org/10.1016/j.jcot.2019.06.015.
11. Benanti E, De Santis G, Leti Acciaro A, Colzani G, Baccarani A, Starnoni M. Soft tissue coverage of the upper limb: a flap reconstruction overview. Ann Med Surg 2020;60:338-43. https://doi.org/10.1016/j.amsu.2020.10.069.
12. Xue X, Li N, Ren L. Effect of vacuum sealing drainage on healing time and inflammation-related indicators in patients with soft tissue wounds. Int Wound J 2021;18(5):639-46. https://doi.org/10.1111/iwj.13565.
13. Han HH, Jun D, Moon S-H, Kang IS, Kim MC. Fixation of split-thickness skin graft using fast-clotting fibrin glue containing undiluted high-concentration thrombin or sutures: a comparison study. SpringerPlus 2016;5(1):1902. https://doi.org/10.1186/s40064-016-3599-x.
14. Braza ME, Fahrenkopf MP. split-thickness skin grafts: StatPearls Publishing, Treasure Island (FL); 2022 2022. [Accessed on: January 25, 2024]. Available from URL:https://www.ncbi.nlm.nih.gov/books/NBK551561/
15. Lee SH, Kim YJ. Effectiveness of double tie-over dressing compared with bolster dressing. Arch Plast Surg 2018;45(3):266-70. https://doi.org/10.5999/aps.2017.01424.
16. Waltzman JT, Bell DE. Vacuum-assisted closure device as a split-thickness skin graft bolster in the burn population. J Burn Care Res 2014;35(5):e338-e42. https://doi.org/10.1097/bcr.0000000000000009.
17. Petkar K, Dhanraj P, Harinatha S. Vacuum closure as a skin-graft dressing: a comparison against conventional dressing. European J Plast Surg 2012;35. https://doi.org/10.1007/s00238-012-0698-y.
18. Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum-assisted closure (VAC) device. Ann Plast Surg 2007;58(5):573-6. https://doi.org/10.1097/01.sap.0000237638.93453.66.
19. Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 2006;244(5):700-5. https://doi.org/10.1097/01.sla.0000217745.56657.e5.
20. Nguyen TQ, Franczyk M, Lee JC, Greives MR, O'Connor A, Gottlieb LJ. Prospective randomized controlled trial comparing two methods of securing skin grafts using negative pressure wound therapy: vacuum-assisted closure and gauze suction. J Burn Care Res 2015;36(2):324-8. https://doi.org/10.1097/bcr.0000000000000089.
21. Svensson-Björk R, Saha S, Acosta S, Gerdtham UG, Hasselmann J, Asciutto G, et al.. Cost-effectiveness analysis of negative pressure wound therapy dressings after open inguinal vascular surgery - The randomised INVIPS-Trial. J Tissue Viability 2021;30(1):95-101. https://doi.org/10.1016/j.jtv.2020.09.005.
22. Älgå A, Löfgren J, Haweizy R, Bashaireh K, Wong S, Forsberg BC, et al. Cost analysis of negative-pressure wound therapy versus standard treatment of acute conflict-related extremity wounds within a randomized controlled trial. World J Emerg Surg 2022;17(1):9. https://doi.org/10.1186/s13017-022-00415-1.
|
CONFLICT OF INTEREST Authors declared no conflict of interest, whether financial or otherwise, that could influence the integrity, objectivity, or validity of their research work. GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |