![]() https://doi.org/10.35845/kmuj.2023.22956 ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2023.22956 ORIGINAL ARTICLE
Indexed stroke volume in children with varying left ventricle ejection fraction and its correlation with various cardiac factors
Rizwanullah1,
Ahmad Usaid Qureshi 1 ![]() ,
Syed Najam Hyder 1, Masood Sadiq 1
,
Syed Najam Hyder 1, Masood Sadiq 1
|
1: Department of Pediatric Cardiology, The Children’s Hospital and University of Child Health Sciences, Lahore, Pakistan
Email
Contact #: +92-333-4485306 Date Submitted: July 03, 2022 Date Revised: January 21, 2023 Date Accepted: February 07, 2023 |
|
THIS ARTICLE MAY BE CITED AS: Rizwanullah, Qureshi AU, Hyder SN, Sadiq M. Indexed stroke volume in children with varying left ventricle ejection fraction and its correlation with various cardiac factors. Khyber Med Univ J 2023;15(1):31-7. https://doi.org/10.35845/kmuj.2023.22956 |
ABSTRACT
OBJECTIVE: To determine the variation in indexed stroke volume (LVSVi) in children with varying left ventricle ejection fraction (LVEF) using cardiac magnetic imaging (CMR) and its correlation with various cardiac factors.
METHODS: This observational comparative study was conducted at The Children’s Hospital, Lahore, Pakistan from December 2018 to November 2021. All children below 18 years’ age presenting to hospital for CMR for tissue characterization, having normal vital organs function and no clinical signs of heart failure were included in the study. Relevant clinical data was recorded. CMR was performed using 1.5T Philips Ingenia MRI scanner. The data were analyzed with varying LVEF and correlation of LVSVi with various cardiac factors including indexed left ventricular end diastolic volume (LVEDVi), cardiac output (CO) and heart rate (HR).
RESULTS: Out of 175 patients, 170 children up to 18 years old completed the test with mean age 14.3±3.3 years. Mean LVSVi was 42+12 ml/m2 which followed Frank Starling curve except in children with LVEF <36%. Mean LVEDVi was 86±34 ml/m2. LVSVi did not correlate with heart rate or indexed ventricular systolic volumes acting as an independent variable. Minimum LVSVi remained similar all groups as demonstrated through centile distribution.
CONCLUSION: Indexed stroke volume is an independent variable in children having normal vital organs function with varying LVEF. It can serve as an independent monitoring parameter for clinical management of children with impaired ejection fraction.
KEYWORDS: Stroke Volume (MeSH); Blood (MeSH); Heart (MeSH); Ventricular Function (MeSH); Cardiac Output (MeSH); Cardiac Output, high (MeSH); Cardiac Output, low (MeSH); Child (MeSH); Child, Preschool (MeSH); Cardiac MRI (Non-MeSH); Cardiac Imaging Techniques (MeSH); Magnetic Resonance Imaging (MeSH).
INTRODUCTION
Cardiovascular system in humans is based on an active pump in the form of four chambered heart pushing blood through a network of vessels. The end point of this system is to provide end organs optimal blood volume at an optimal perfusion pressure to allow oxygen, carbon dioxide, nutrition and waste exchange. This aim is achieved by the heart altering its rate, amount of blood pumped with each contraction (stroke volume) and optimal contractility. Under certain conditions, this ability is hampered and optimal cardiac output is not generated leading to heart failure, low cardiac output syndrome, syncope or shock in addition to adversely affecting vital organs function including deranged renal profile, pulmonary edema and altered conscience level. Under circumstances with impaired contractility and limited available heart rate range especially in children, understanding the variation in stroke volume is of utmost importance.1
Conditions with impaired left ventricle (LV) contractility are a leading non-infectious cause of death in children over 1 year of age. These mainly include idiopathic, familial or post viral dilated cardiomyopathies (DCM) and acute myocarditis. The prognosis is guarded in a large fraction of these children and invariably needs anti-failure medication.2 All the medications are aimed at improving stoke volume and cardiac output using Frank Starling curve estimations. As the disease progresses, many children require a heart transplant.2 While awaiting heart transplant, these children require bridge therapies. These may include extracorporal life support (ECMO), cardiac resynchronization therapy (CRT), continuous flow devices, LV assist devices (LVAD) or intra-aortic balloon pump (IABP) with good one-year survival rate.3 Aiming for “normal for age” stroke volume and cardiac output, pushes pharmacological agents’ doses as well as bridge therapy devices settings too high. As a consequence, such efforts can deplete the reserves rapidly without providing any added benefit. Settings aimed at producing minimum stroke volume able to sustain normal vital organ function can result in major prolongation of bridging therapy. It leads to better opportunity of receiving a heart transplant. The minimum volume of blood required per beat (LV stroke volume, LVSV) in children with low ejection fraction to sustain vital organ functions still remains undefined.4 The present recommendations for settings and rates in such bridging therapies as well as pharmacological agents are dependent on adult data and hence may result in over-performance, utilizing additional unnecessary resources and limiting the bridging therapy time.5
Cardiac magnetic resonance imaging (CMR) is advanced technique, considered a gold standard for left and right ventricular volume and flow assessment.6 CMR in children is in evolutionary stage in Pakistan The main challenge in pediatric age group is requirement of actively holding breath for 4-15 seconds multiple times and being stable enough to lie flat for 45-75 min for complete study. Otherwise, general anaesthesia is required for CMR in children. Having pointed out this limitation, it inadvertently implies that any child able to undergo complete CMR study has adequate perfusion to allow a compensated state without causing orthopnea or pulmonary edema while lying flat over extended period of time and still has normal conscience level and respiratory reserve to hold breath whenever asked during study. Renal function tests are also a pre-requisite for Gadolinium contrast injection indicating all these children also have a normal renal perfusion. Overall, all these parameters directly or indirectly imply that all children completing CMR study under conscious spontaneous breathing have normal vital organ functions. This novel concept has not been implied previously for evaluating stroke volume in children with varying LVEF.
So, in this study, we aimed to find the variation in stroke volume with varying in vivo setting in children with normal vital organ functions, utilizing CMR effect of various cardiac factors on LVSVi. It could, as a result, help maintaining a minimum stroke volume required to sustain vital organ functions in such children despite varying left ventricle ejection fraction (LVEF).
METHODS
This observational comparative study was conducted at The Children’s Hospital, Lahore, Pakistan from December 2018 to November 2021, after obtaining ethical approval from institutional review board. All children below 18 years’ age presenting to hospital, already booked for CMR for tissue characterization having normal renal function test, liver function test, with no clinical signs of heart failure were included in the study, after obtaining a written consent from the parents. Children with congenital heart defect or chronic lung disease were excluded from the study. Normal vital organ functions, defined as normal renal profile (normal blood urea nitrogen and serum creatinine), liver function (normal alanine transaminase, alkaline phosphatase), neurological status (conscious, alert, following commands), respiratory (normal breathing, normal oxygen saturation in room air, able to intermittently hold breath for CMR image acquisition) and cardiovascular system (New York Heart Association Class I or II, able to lie flat for more than 30 minutes, normal peripheral pulses and warm peripheries with no inotropic support) were documented as a perquisite for inclusion in the study. All these parameters were already a pre-requisite for CMR according to hospital protocols. Hence, all children enrolled in the study already did comply with operational definition of normal vital organ function. CMR was performed in all these children using 1.5T Philips Ingenia MRI scanner for documenting LV status in terms of function, volume and tissue characterization as per institutional protocol.6 CMR when performed without anesthesia usually requires around 45 to 75 minutes lying flat on scanner table and frequent breath holds ranging from 4 to 10 seconds for Cine Steady State Free Precession (SSFP) CMR images. This limitation of CMR without anesthesia was invariably utilized as standard for documenting a clinically normal cardiac and respiratory status.6 Considering 10% incidence of low cardiac output in various conditions in children, hypothesized frequency of outcome factor in the population (p) 5% ±5, confidence limits 5% and design effect 1, using formula sample size n = [DEFF*Np(1-p)]/ [(d2/Z21-α/2*(N-1)+p*(1-p)], sample size was calculated to be 170 with confidence level 97% with OpenEpi, Version 3, open source calculator.7 Volumes were calculated using stacks of cine Steady-State Free Precession images in short and long axis. Flow studies were performed using phase contrast (PC) imaging. To limit any motion or rate disparity, all sequences were recorded at 25 phases per cycle. Post processing was performed through PHILIPS IntelliSpace portal and confirmed using Medis MR suite Qf low and QMass software. Cardiac factors included all cardiac physiological parameters calculated during routine CMR including right and left ventricular systolic and diastolic volumes (LVEDV/RVEDV/LVESV/RVESV), left ventricular ejection fraction (LVEF), Heart rate (HR), Cardiac output (CO) and Cardiac Index (CI). Factors were also indexed to body surface area for detailed evaluation.
The data was entered in SPSS v. 20 and analyzed using its statistical software. Mean with standard deviation was calculated for normally distributed data confirming through Shapiro-Wilk test considering small sample size. Frequencies were calculated for nominal data. Pearson and Spearman correlation were calculated for quantitative and qualitative data respectively. Cardiac function as analyzed in terms of LVEF and children were further categorized as normal or borderline low (EF >50%), impaired (EF 36-49%) and severely impaired (EF <35%) for further evaluation.8 Student’s T test was used to find statistically significant difference in variables among three sub groups according to ejection fraction considering p<0.05 as significant. Correlation was calculated between LVSVi and various cardiac factors. Multivariate analysis was performed to examine significant cardiac factors affecting LVSVi.
RESULTS
A total 175 patients, up to 18 years old, were enrolled in the study during the specified duration. Three patients could not complete the study due to arrhythmias while two had severe claustrophobia. After exclusion of these 5 cases, 170 were finally included in the data analysis. Demographic profile was tabulated [Table 1].
Table I: DEMOGRAPHIC PROFILE OF THE STUDY PARTICIPANTS
|
Variables |
Total patients (N=170), [mean+SD] |
Borderline low or normal LV function group (n=120), [mean+SD] |
Impaired LV function group (n=37), [mean+SD] |
Severely impaired LV function group (n=13), [mean+SD] |
p value |
|
Age [years] |
14.3±3.3 |
14.3±3.2 |
14.9±3.2 |
13.2±3.8 |
0.52 |
|
Male (n, %) |
121 (71%) |
84 (70%) |
26 (70%) |
11 (85%) |
0.6 |
|
Weight [kg] |
51.2±19 |
51.2±18.8 |
52.8±18.7 |
47.5±21.5 |
0.18 |
|
Height [m] |
1.56±0.2 |
1.57±0.19 |
1.56±0.19 |
1.48±0.28 |
0.26 |
|
Surface area [m2] |
1.47±0.36 |
1.47±0.35 |
1.49±0.36 |
1.39±0.43 |
0.11 |
|
Heart rate [Beats Per Minute] |
79±15 |
77±14 |
82±15 |
84±21 |
0.23 |
|
Test duration [minutes] |
65±13 |
64±13 |
66±12 |
73±14 |
0.56 |
SD: standard Deviation; LV: left Ventricle; m: meters; kg: Kilogram; %: percentage
There was no significant difference in age, height, weight, surface area or hear rate between either gender (p=0.12). Left and right ventricular volumetric analysis showed varying impairment in cardiac function on basis of ejection fraction (EF) [Table 11]. Children were further analyzed as normal or borderline low (EF >50%), impaired (EF 36-49%) and severely impaired (EF <35%) LV systolic function subgroups based on operational definition.8 Cardiac performance in terms of pulmonary and systemic stroke volume and cardiac output varied significantly with EF [Table 11].
Table II: VENTRICULAR DIMENSIONS AND CARDIAC FACTORS IN THE STUDY PARTICIPANTS
|
Variables |
Total patients (N=170), [mean + SD] |
Borderline low or normal LV function group (n=120), [mean + SD] |
Impaired LV function group (n=37), [mean + SD] |
Severely impaired LV function group (n=13), [mean + SD] |
p value |
|
|
LV Volumetric assessment |
LV Indexed end diastolic volume, [LVEDVi, ml/min/m2] |
86±34 |
81±24 |
78±20 |
149±68 |
<0.001 |
|
LV Indexed end systolic volume, [LVESVi, ml/min/m2] |
42±31 |
33±12 |
43±12 |
118±65 |
<0.001 |
|
|
LV ejection fraction, [LVEF, %] |
54±12 |
60±7 |
44±4 |
24±9 |
<0.001 |
|
|
Indexed LV ejection fraction, [LVEFi/m2, %] |
39±16 |
44±15 |
32±11 |
18±7 |
<0.001 |
|
|
RV Volumetric assessment |
RV Indexed end diastolic volume [RVEDVi, ml/min/m2] |
118±48 |
113±45 |
129±39 |
140±79 |
0.65 |
|
RV Indexed end systolic volume [RVESVi, ml/min/m2] |
59±37 |
52±29 |
69±29 |
104±76 |
<0.001 |
|
|
RV ejection fraction [RVEF, %] |
52±13 |
56±11 |
47±11 |
30±13 |
<0.001 |
|
|
Indexed RV ejection fraction [RV EFi/m2, %] |
38±16 |
41±16 |
34±13 |
25±16 |
0.001 |
|
|
Cardiac output parameters |
LV Stroke volume [ml] |
61±20 |
66±19 |
50±18 |
41±11, |
0.001 |
|
RV Stroke volume [ml] |
62±20 |
67±20 |
55±18 |
41±11 |
<0.001 |
|
|
Indexed LV Stroke volume [ml/m2] |
42 ±12 |
46±12 |
34±10 |
31±7 |
<0.001 |
|
|
Indexed RV Stroke volume [ml/m2] |
43±13 |
46±13 |
37±11 |
31±7 |
<0.001 |
|
|
Cardiac output [CO, L/min] |
4.7±1.5 |
5.0±1.4 |
4.0±1.4 |
3.3±0.7 |
<0.001 |
|
|
Cardiac index [CI, (L/min)/m2] |
3.2±1.1 |
3.5±1.2 |
2.7±0.9 |
2.5±0.8 |
<0.001 |
|
SD: standard Deviation; LV: left Ventricle; RV: Right ventricle; m: meters; %: percentage
LV end diastolic volumes (LVEDV) in children with impaired LV function remained relatively similar to normal LVEF children group though increased significantly as EF fell below 36% in severely impaired LV function category. When indexed to surface area, LVEDVi was increased in 8/13 (62%) children with severely impaired LV function against those with the rest of children (20/157, 12.7%), p<0.001. LV end systolic volumes (LVESV) also showed similar trends though the increase in volumes showed a more gradual progression from normal to severely impaired LV function group. LVESV was increased in only 9/120 (7.5%) in normal children, increased to 10/37 (27%) in children with impaired LV function while nearly all children (12/13, 92%) with severely impaired LV function had marked increase in LVESV, p<0.001. LV stroke volume showed significant decline with LV function impairment, p<0.001. RV end diastolic volume (RVEDV) was slightly higher in children with impaired or severely impaired LV function though the difference remained insignificant, p=0.21. Even when indexed to surface area, RVEDVi remained similar in all groups p=0.65. Indexed RV end systolic volume (RVESVi) was significantly increased in children with severely impaired and impaired LV function (44/50, 88%), p<0.001. Consequently, RV stroke volume was also significantly decreases in impaired LV function group (n=24/37, 65%) and severely impaired LV function group (n=11/13, 84%), p<0.001. Cardiac output decreased significantly with impairment in LV function, p<0.001. Cardiac index was significantly lower in impaired and severely impaired LV function groups though remained relatively similar between themselves (p<0.001).
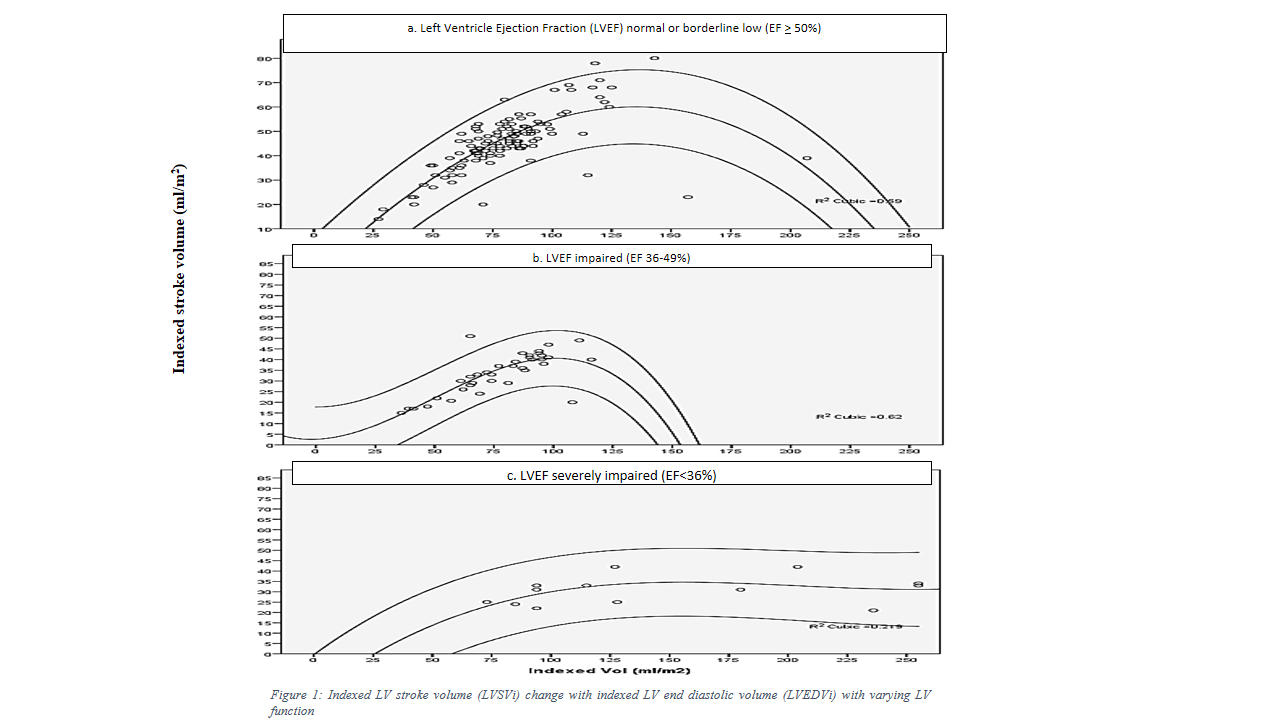
Indexed stroke volume followed Frank Starling curve in children with normal EF, was damp in children with impaired LV function and did not follow the curve in children with severely impaired EF. This finding was further evident from LV dilatation as a measure of improving stroke volume as long as the impairment was not severe. As the LV function deteriorated, marked LV dilatation ensued with no improvement in stroke volume, [Figure 1].
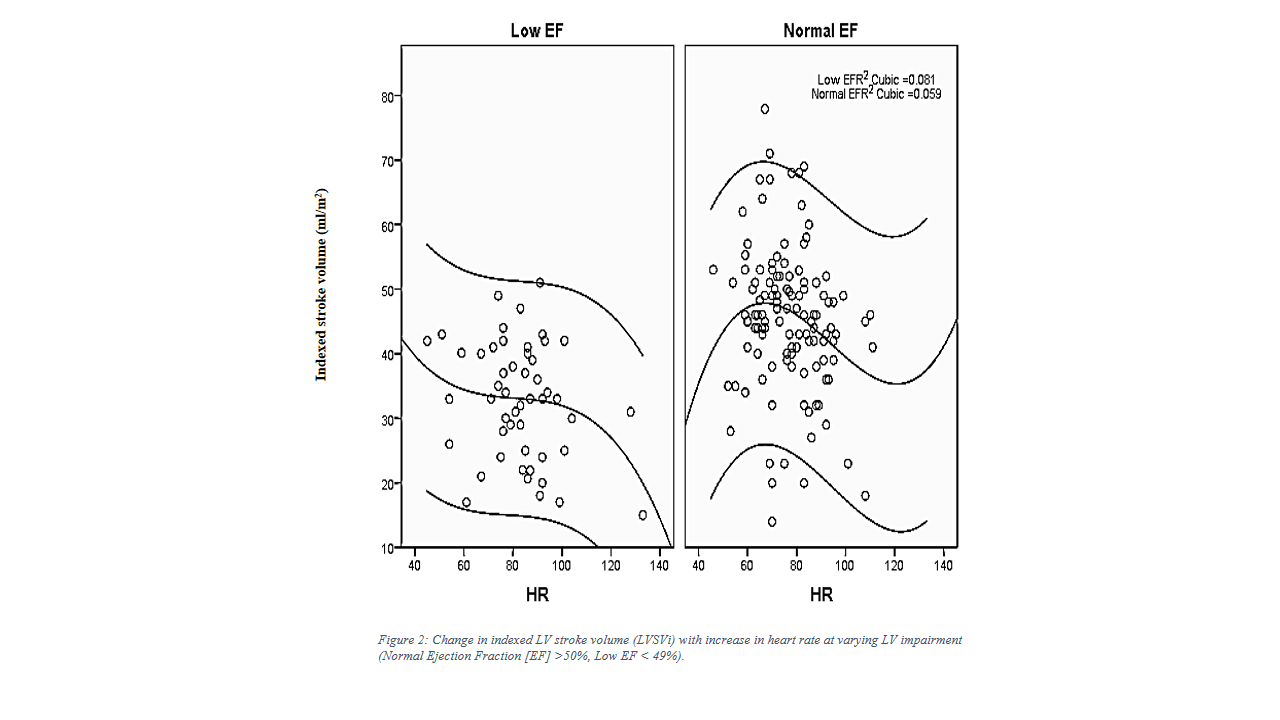
Similarly, low EF patients (EF <49%) did not show increase in stroke volume with increase in heart rate (HR), [Figure 2].
Centiles were calculated for indexed stroke volume in children with normal (LVEF >50%) and low LVEF (<49%) to demonstrate the dispersion of values [Figure 3].
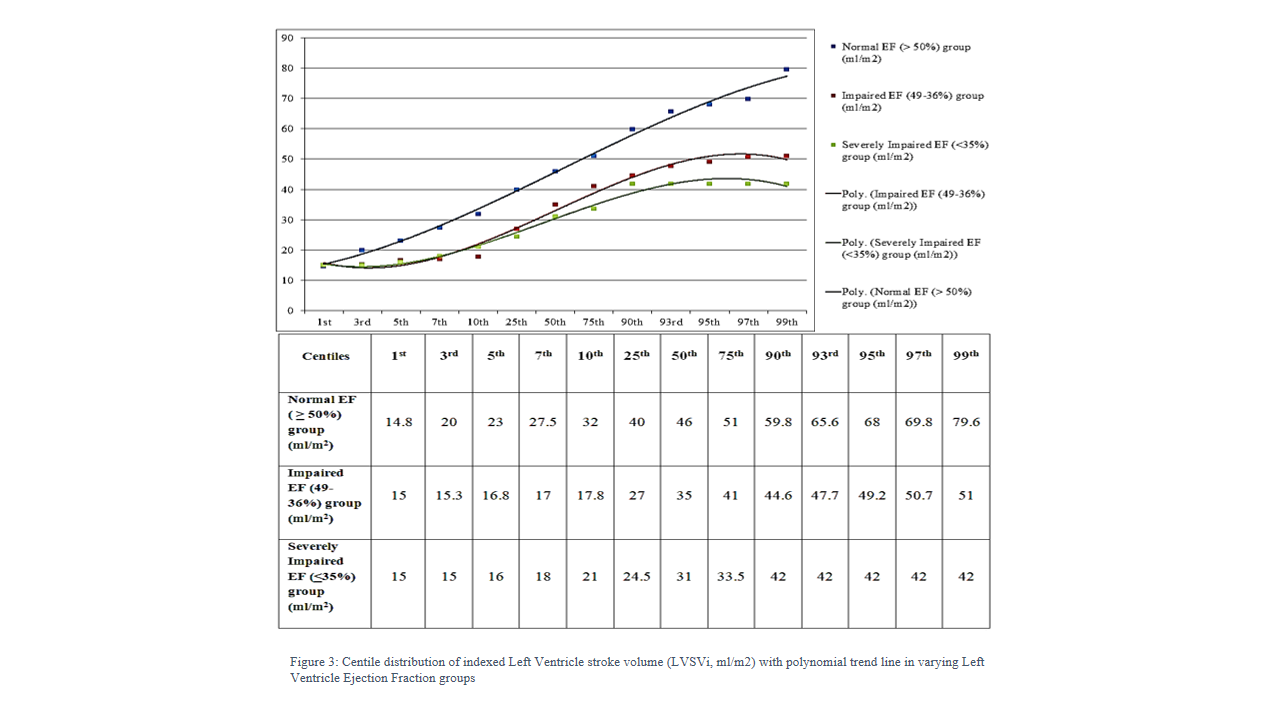
Indexed stroke volume (LVSVi) decreased with decrease ejection fraction however; the lower limits of normal curve in normal and impaired LV function group remained similar. Lower limits of indexed stroke volume normal curve remained 23ml at -2SD and 15ml at -3SD in both normal and low EF groups.
LVSVi did not correlate with heart rate in children with low EF (Pearson’s correlation R -0.24, p=0.09) unlike the significant correlation between stroke volume and heart rate in children with normal EF (Pearson’s correlation R -0.2, p=0.16). Similarly, LVSVi did not correlate with LV or RV parameters, including LVEDVi, LVESVi, RVEDVi, RVESDi) (p=0.09-0.84). Multivariate analysis did not find any factor affecting LVSVi significantly (p=0.06-0.42)).
DISCUSSION
Stroke volume plays an important role in maintaining vital organs perfusion. Any acute or acute on chronic deterioration results in a cascade of events termed as low cardiac output syndrome culminating into multi-organ failure.9 Stroke volume is independent of various cardiac parameters and is mainly dictated by actual cardiac contraction strength.10
It has been documented previously that pediatric age group cardiac output is heart rate dependent as stroke volume does not increase by increasing LV relaxation.11 Our study also demonstrated that stroke volume did not correlate with LV end diastolic volumes. Our study did point out some increase in heart dimensions with decreasing ejection fraction. These findings were similar to previously published adult data although the mechanism of acquisition of stroke volume was variable and mostly based on arterial pulseform.12 Such estimations are not exact and cannot be directly compared to our data. Moreover, this finding overlaps with findings from adult population, probably representing a transitional older population within pediatric age group in our study population.
The children with normal ejection fraction did follow Frank Starling curve. As the LV impairment became severe, stroke volume showed no improvement with increase in LV indexed volume.4 It might be a reflection of inherent myocardial status or demonstration of a burnt out dilated heart that is not able remodel for improved stroke volume. Knock-in animal models have documented this finding previously highlighting a similar pathophysiology.13
Heart rate also seemed to play its positive role in healthy children, though in children with impaired function, any increase in heart rate paradoxically reduced cardiac output through lower stroke volume. The main impact of increased heart rate in children with impaired LV function was due to lesser diastolic filling time with smaller end diastolic volume. This finding also promotes good clinical practices by emphasizing limiting use of chronotropic agents during management of such patients and not allowing undue tachycardia.14
The lower limits of normal distribution curve for stroke volume was very similar in all groups irrespective of ejection fraction [Figure 3]. Stroke volume can be calculated through echocardiography by measuring LV outflow tract flow acceleration15 Echocardiographic measurements correlate with Cardiac MRI findings showing fairly high sensitivity and specificity.16 With availability of centile chart developed in this study, emphasis may shift from following blood pressure to echocardiography based bed side stroke volume assessment.17 Measuring stroke volume can provide a much more objective parameter guiding clinical management.18 It can hence, avoid over and under-treatment in children with compensated heart failure.
The stroke volume centiles can also help in managing advanced heart failure patients on LV assist devices, intra-aortic balloon pump and even extracorporeal life support.19 These values can guide the management protocols for optimal organ perfusion without pushing for unnecessary extra output.20,21
It was a pilot observational comparative study to document stroke volume in children with varying LV function to maintain a compensated state. Being a novel aspect of research, there has been scarcity of previously published data for comparison. Further experimental cohort studies are required to confirm these findings by evaluating changes in cardiac output in patients at varying clinical stages and individually assess the role of anti-failure medications as well as various bridge therapies.
CONCLUSION
Indexed LV Stroke volume follows Frank Starling curve in children with normal LV function. The curve fails to hold in children with severely impaired function. Indexed LV Stroke volume acts as an independent variable in children with impaired LV function. Heart rate and LVEDV do not correlate with stroke volume. Hence, LVSVi can serve as a guiding parameter for clinical management of children with impaired ejection fraction in terms of increasing or decreasing medications to achieve optimal vital organs perfusion avoiding undue adverse effects of medications.
REFERENCES
1. Halperin HR, Tsitlik JE, Guerci AD, Mellits ED, Levin HR, Shi AY, et al. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation 1986;73(3):539-50. https://doi.org/10.1161/01.cir.73.3.539
2. Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, et al. Dilated cardiomyopathy. Nat Rev Dis Primers 2019;5(1):32. https://doi.org/10.1038/s41572-019-0084-1
3. Reineke DC, Mohacsi PJ. New role of ventricular assist devices as bridge to transplantation: European perspective. Curr Opin Organ Transplant 2017;22(3):225-30. https://doi.org/10.1097/mot.0000000000000412
4. Sigurdsson TS, Lindberg L. Indexing haemodynamic variables in young children. Acta Anaesthesiol Scand 2021;65(2):195-202. https://doi.org/10.1111/aas.13720
5. Young DB. Control of Cardiac Output. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Introduction. [Accessed on: February 5, 2021]. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK54473/
6. Patel AR, Kramer CM. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc Imaging 2017;10(Pt A):1180-93. https://doi.org/10.1016/j.jcmg.2017.08.005
7. Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol 2008;31(8):388-91. https://doi.org/10.1002/clc.20262
8. Harkness A, Ring L, Augustine, DX, Oxborough D, Robinson S, Sharma V. Normal Reference Intervals for Cardiac Dimensions and Function for Use in Echocardiographic Practice: A Guideline from the British Society of Echocardiography. Echo Res Pract 2020;7(1):G1-18. https://doi.org/10.1530/erp-19-0050
9. Massé L, Antonacci M. Low cardiac output syndrome: identification and management. Crit Care Nurs Clin North Am 2005;17(4):375-83. https://doi.org/10.1016/j.ccell.2005.07.005
10. Backer DD. Stroke volume variations. Minerva Anestesiol 2003;69(4):285-8.
11. Erez E, Mazwi ML, Marquez AM, Moga MA, Eytan D. Hemodynamic patterns before inhospital cardiac arrest in critically Ill children: An exploratory study. Crit Care Explor. 2021;3(6):e0443. https://doi.org/10.1097/cce.0000000000000443
12. Kerkhof PLM, de Ven PMV, Yoo B, Peace RA, Heyndrickx GR, Handly N. Ejection fraction as related to basic components in the left and right ventricular volume domains. Int J Cardiol 2018;255:105-10. https://doi.org/10.1016/j.ijcard.2017.09.019
13. Inoue T, Kobirumaki-Shimozawa F, Kagemoto T, Fujii T, Terui T, Kusakari Y, et al. Depressed Frank-Starling mechanism in the left ventricular muscle of the knock-in mouse model of dilated cardiomyopathy with troponin T deletion mutation ΔK210. J Mol Cell Cardiol. 2013;63:69-78. https://doi.org/10.1016/j.yjmcc.2013.07.001
14. Chew MS. Haemodynamic monitoring using echocardiography in the critically ill: a review. Cardiol Res Pract 2012;2012:139537. https://doi.org/10.1155/2012/139537
15. Jeong H, Lee H, Jung J, Kim H, Yu J, Yoon H, et al. Evaluation of left ventricular function with cardiac magnetic resonance imaging and echocardiography after administration of dobutamine and esmolol in healthy beagle dogs. J Vet Med Sci 2021;83(4):581-91. https://doi.org/10.1292/jvms.18-0703
16. Vinet A, Nottin S, Lecoq AM, Guenon P, Obert P. Reproducibility of cardiac output measurements by Doppler echocardiography in prepubertal children and adults. Int J Sports Med 2001;22(6):437-41. https://doi.org/10.1055/s-2001-16241
17. Aligholizadeh E, Teeter W, Patel R, Hu P, Fatima S, Yang S, et al. A novel method of calculating stroke volume using point-of-care echocardiography. Cardiovasc Ultrasound 2020;18(1):37. https://doi.org/10.1186/s12947-020-00219-w
18. Sabaz MN, Akın A, Bilici M, Demir F, Türe M, Balık H. Factors affecting mortality in children with dilated cardiomyopathy. Turk J Pediatr 2019;61(4):485-92. https://doi.org/10.24953/turkjped.2019.04.003
19. Ferrari G, Molfetta AD, Zieliński K, Fresiello L, Górczyńska K, Pałko KJ, et al. Control of a Pediatric Pulsatile Ventricular Assist Device: A Hybrid Cardiovascular Model Study. Artif Organs 2017;41(12):1099-108. https://doi.org/10.1111/aor.12929
20. Nardo MD, MacLaren G, Marano M, Cecchetti C, Bernaschi P, Amodeo A. ECLS in Pediatric Cardiac Patients. Front Pediatr 2016;4:109. https://doi.org/10.3389/fped.2016.00109
21. Cooper DS, Jacobs JP, Moore L, Stock A, Gaynor JW, Chancy T, et al. Cardiac extracorporeal life support: state of the art in 2007. Cardiol Young 2007;17 Suppl 2:104-15. https://doi.org/10.1017/s1047951107001217
|
Following authors have made substantial contributions to the manuscript as under:
Ri & AUQ: Acquisition, analysis and interpretation of data, drafting the manuscript, critical review, approval of the final version to be published SNH & MS: Concept and study design, critical review, approval of the final version to be published
Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |