![]() https://doi.org/10.35845/kmuj.2023.22778 ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2023.22778 ORIGINAL ARTICLE
Glasgow Blatchford scoring system enables accurate risk stratification of patients with upper gastrointestinal haemorrhage
Fatima
Khalil 1, Nayyar Yaqoob 1, Shahida Perveen 1,
Humera Qureshi 2,3, Muhammad Imran Khan2,3 ![]()
|
1: General Medicine, Fauji Foundation Hospital, Rawalpindi, Pakistan 2: Department of Mathematics and Statistics, The University of Haripur, Haripur, Khyber Pakhtunkhwa, Pakistan 3: Department of Epidemiology and Biostatistics, Anhui Medical University, China Email Contact #: +92-333-9268217 Date Submitted: April 25, 2022 Date Revised: September 12, 2023 Date Accepted: September 19, 2023 |
|
THIS ARTICLE MAY BE CITED AS: Khalil F, Yaqoob N, Perveen S, Qureshi H, Khan MI. Glasgow Blatchford scoring system enables accurate risk stratification of patients with upper gastrointestinal haemorrhage. Khyber Med Univ J 2023;15(3):148-54. https://doi.org/10.35845/kmuj.2023.22778 |
ABSTRACT
OBJECTIVE: To evaluate Glasgow Blatchford (GB) scores ability for risk stratification in patients presenting with upper gastrointestinal bleeding (UGIB).
METHODS: The prospective cohort study was conducted in the inpatient department of medicine at Fauji Foundation Hospital Rawalpindi, Pakistan, from April to September 2021. One hundred and thirty patients with UGIB (hematemesis, melena, and blood in the nasogastric tube) were included by consecutive sampling technique. We excluded traumatic patients with UGIB, pregnant females, patients with chronic kidney disease, anorexia nervosa, bulimia nervosa, and chronic diarrhea. Laboratory and demographic data were collected. The GB score was calculated at the time of admission. Data was analyzed through SPSS version 23, and frequencies were deduced. Groups were compared using the chi-square test.
RESULTS: Mean age of patients was 61.1±13.8 years. There were 56 (43.1%) males and 74 (56.9%) females in the study. The main reason for acute gastrointestinal bleeding was Hepatitis C-associated portal hypertension (n = 103; 79.2%), followed by non-steroidal anti-inflammatory drug-induced gastrointestinal bleeding (n=13; 10.0%). There were 90 (69.2%) patients in high-risk group (Group A) and 40 (30.8%) in low-risk group (Group B). The high-risk group had a significantly higher GB score than the low-risk group (11.61±3.2 vs 3.85±1.9, p<0.001). GB score of ≥4 has sensitivity of 97.7%, a specificity of 92.5%, and an area under curve of 0.967 with a p-value of<0.001.
Conclusion: GB score has an excellent accuracy for risk stratification of patients with UGIB. With a cutoff of ≥4, GB score accurately identifies 97.7% of high risk patients.
KEYWORDS: Bleeding (MeSH); Gastrointestinal bleeding (Non-MeSH); Gastrointestinal Hemorrhage (MeSH); Endoscopy (MeSH); Varices (MeSH); Varicose Veins (MeSH); Esophageal and Gastric Varices (MeSH).
INTRODUCTION
Upper gastrointestinal bleeding refers to bleeding caused by all causes involving the area above the ligament of treitz. 1,2 It is a common condition that may lead to high morbidity and mortality rates.3 The mortality rate is documented as two to fifteen percent for cases of upper gastrointestinal bleeding.4 This may increase with increasing age due to the use of non-steroidal anti-inflammatory drugs and other co-morbidities. Chief causes include variceal (portal hypertensive gastropathy and hypertensive gastropathy) and non-variceal bleeds (gastritis, peptic ulcer disease, esophagitis, tumors, and Mallory-Weis syndrome).1
Generally, cases of upper gastrointestinal bleeding involve the admission of the patients. Hence, knowing which grade or degree of bleed requires attention is vital to decreasing the management burden on the doctors and hospital. It is necessary to give attention to the deserving critical patients. Up to 80% of bleeds may recover spontaneously, so knowing when to refer or admit the patient is important. Many scoring systems may help identify the severity of bleeds and at-risk patients, including Rock-All scoring and Glasgow Blatchford (GB) scoring. However, the efficacy of any of these scores in predicting the outcomes adequately is still unclear.5,6 The GB scoring involves comparison of clinical findings and laboratory tests to identify at-risk individuals and hence appears to be more practical in emergencies. 7
A study by Islam MS et al.,6 documented that GB scoring predicts low-risk and high-risk individuals quite accurately. Similarly, another study from Korea, explaining the risk stratification of upper gastrointestinal bleed patients, concluded that GB scoring is effective in predicting the need of intervention and risk identification. This decreases hospital expenditure and the burden of disease.8,9 Also, unnecessary hospital admissions may decrease patient anxiety about the disease. Contrarily, a study demonstrated that albumin, international normalized ratio, mental status, systolic blood pressure, and age 65 score (AIMS65) can also predict the risk equally in upper gastrointestinal bleed patients, but they are more sensitive in finding the mortality rate. 10
Since the emergency patient influx with gastrointestinal bleeds is quite high in our region, correct identification of patients at high risk can help in prompt management and decrease in mortality rate. The identification of low-risk patients can ensure early discharge and assurance for the patients, decreasing their disease anxiety. Most of the studies examining the GB score’s role in UGIB are done in Caucasians. There is limited data for our population, which is unique to Caucasians. So, we planned this study to evaluate if GB scoring can successfully predict the risks in our population and to validate a more effective and clinically practical scoring system to identify at-risk patients in emergencies. The objective of this study was to establish the accuracy of GB scoring system for identifying the risk of gastrointestinal haemorrhage in patients of Fauji Foundation Hospital Rawalpindi, Pakistan.
METHODS
This prospective cohort study was conducted in the inpatient department of medicine at Fauji Foundation Hospital Rawalpindi, Pakistan, from April to September 2021. Patients were recruited using the consecutive sampling size technique.
The study was approved by the ethics committee of the Fauji Foundation Hospital Rawalpindi (reference no. 445/RC/FFH/RWP) and informed consent was obtained from all study participants before recruitment. The minimum sample size was calculated to be 120. The sample size was calculated using WHO sample size calculator software based on the sensitivity and specificity of the GB scoring system to predict the need for hospital-based intervention among patients with upper gastrointestinal hemorrhage. Sensitivity of 97%,11 specificity of 48%, 11.0%12 prevalence of upper gastrointestinal bleed, 10% precision, and 20% dropout were used to calculate the minimum required sample size11.
Inclusion criteria: All individuals with an age greater than eighteen years were included. Patients coming to the hospital with hematemesis, melena, and blood in the nasogastric tube were considered upper gastrointestinal bleeding patients and were included in the study.
Exclusion criteria: Following individuals were excluded: traumatic patients presenting with gastrointestinal bleeding, all pregnant females, those with chronic kidney disease, anorexia nervosa, bulimia nervosa and chronic diarrhea.
The data was prospectively collected. Data was collected on a form with laboratory parameters and patients’ particulars. The GB score was calculated at the time of admission. Patients were classified into high-risk and low-risk groups based on clinical, therapeutic, and endoscopic characteristics. Patients were followed during admission, and all patients requiring transfusion of blood, having grade 3 and 4 varices on endoscopy, requiring endoscopic intervention, ICU admission, and death as outcome were included as high-risk patients (Group A). Patients not requiring the above-mentioned treatments and patients who were discharged successfully were labelled as low-risk (Group B). The
GB score was compared between these two groups. Rebleed was considered as any bleeding, with endoscopic evidence after the third day of treatment for bleeding. All emergency management and intervention decision were made by a gastroenterologist. Data was entered in SPSS version 23, and frequencies were deduced. Groups were compared using the chi-square test. Receiver operator characteristic (ROC) curve analysis was carried out to define the cut-off values of GB score along with sensitivities and specificities. GB score levels were stratified for additional clinical risk factors including blood transfusion, ICU admission, and death (yes vs no); endoscopy (normal vs abnormal) and grade of varices (1/2 vs 3/4).
RESULTS
One Hundred and Thirty patients presenting with upper gastrointestinal bleeding in the emergency department were included in this study, with mean age of 61.1±13.8 years (age range 18 – 92 years). The main reason for acute gastrointestinal bleeding was Hepatitis C associated portal hypertension 103 (79.2%), followed by non-steroidal anti-inflammatory drug induced gastrointestinal bleeding (10.0%). There were 90 (69.2%) patients belonging to high-risk group (Group A) whereas 40 (30.8%) belonged to low risk group (Group B). Demographic and clinical characteristics of high and low risk patients are summarized in table 1. High risk patients had significantly higher mean GB score than low risk patients. One quarter of the high risk patients needed intensive care unit admission, and similar proportion died.
Around 67.7% (n = 88) patients underwent endoscopic examination. Out of these, 61 (69.3%) were high-risk patients, while 27 (30.6%) were low-risk patients. Table 1 gives details of endoscopic findings among high- and low-risk groups. Endoscopy was abnormal in more than 98% of the high-risk group in contrast to three-quarters of the low-risk group (p = 0.001). The most common endoscopic finding in the high-risk group was esophageal and gastric varices (68.8%), where all the varices were of grade III and IV, followed by ulcers (11.4%) and portal gastropathy (11.4%).
Figure 1 gives the distribution of high risk and low risk patients at individual GBS score. The ROC curve analysis demonstrated that GB score is an excellent tool to predict and differentiate between high and low risk patients presenting with acute gastrointestinal bleeding, where the area under the curve (AUC) was 0.967 with 95% CI of 0.93 – 0.99 (p<0.001) as shown in figure 2.
The results of ROC curve analysis suggested GBS score to be a good estimate of ICU admission or death in high risk patients upper gastrointestinal bleeding patients if no intervention is provided on time. Table II shows sensitivity and specificity of various cut-offs for GBS score. The score of ≥4 is found to be a suitable cutoff value where sensitivity is 97.7% (95% CI 95.0 – 100%) and specificity is 92.5% (95% CI 89.0 – 95.5%). Using this cutoff value, it is possible to detect 97.7% of the high risk patients and 92.5% of low risk patients accurately.
The mean GB score comparison with respect to risk factors including blood transfusion, grade III/IV varices, endoscopic intervention, ICU admission and outcome is given in table III. A significantly higher GB score was noted for all risk factors, except for grade III/IV varices (p=0.824).
Table I: Comparison of demographic and clinical characteristics of high and low risk patients (n=130)
|
Characteristics |
Overall n=130 |
Levels of risk |
P-values |
|||
|
High Risk Patients (Group A) n=90 |
Low Risk Patients (Group B) n=40 |
|||||
|
Age in years (mean±SD) |
61.09±13.8 |
59.9±13.9 |
63.6±13.4 |
0.159 |
||
|
Gender n (%) |
Male |
56 (43.1%) |
37 (41.1%) |
19 (47.5%) |
0.497 |
|
|
Female |
74 (56.9%) |
53 (58.9%) |
21 (52.5%) |
|||
|
Bleeding Risk factors n (%) |
HCV portal hypertension |
103 (79.2%) |
74 (82.2%) |
29 (72.5%) |
0.092 |
|
|
Non-HCV portal hypertension |
4 (3.1%) |
3 (3.3%) |
1 (2.5%) |
|||
|
NSAIDs |
13 (10.0%) |
5 (5.6%) |
8 (20.0%) |
|||
|
Anticoagulants |
3 (2.3%) |
3 (3.3%) |
0 (0%) |
|||
|
Bernard–Soulier syndrome |
1 (0.8%) |
1 (1.1%) |
0 (0%) |
|||
|
Pancytopenia |
1 (0.8%) |
1 (1.1%) |
0 (0%) |
|||
|
Esophageal Cancer |
2 (1.5%) |
1 (1.1%) |
1 (2.5%) |
|||
|
Ulcerative colitis |
3 (2.3%) |
1 (1.1%) |
2 (5.0%) |
|||
|
Bleeding duration (hours) (mean±SD) |
41.17±43.4 |
47.18±47.6 |
27.6±20.5 |
0.019 |
||
|
Laboratory findings n (%) |
Hemoglobin |
9.04±3.0 |
7.68±2.4 |
12.07±1.6 |
<0.001 |
|
|
WBC |
8.52±5.0 |
8.29±5.7 |
9.03±2.9 |
0.442 |
||
|
Platelets Bilirubin |
146.76±105.2 25.60±32.64 |
127.97±96.6 22.86±29.9 |
188.58±112.4 31.78±37.6 |
0.002 0.151 |
||
|
ALT |
53.35±34.2 |
53.63±35.5 |
52.73±31.5 |
0.890 |
||
|
ALP |
203.73±139.7 |
179.23±73.7 |
258.85±218.3 |
0.002 |
||
|
Albumin |
29.02±7.1 |
27.88±7.2 |
31.5±6.1 |
0.006 |
||
|
PT |
4.78±10.2 |
6.08±11.9 |
1.88±3.1 |
0.031 |
||
|
APTT |
7.30±14.1 |
9.15±16.4 |
3.20±4.6 |
0.027 |
||
|
INR |
1.36±0.74 |
1.45±0.8 |
1.15±0.2 |
0.033 |
||
|
Urea |
11.10±8.2 |
12.88±9.1 |
7.00±3.2 |
<0.001 |
||
|
Creatinine |
128.18±106.6 |
142.86±124.0 |
94.31±24.1 |
0.017 |
||
|
Blood Sugar Random |
73.54±103.89 |
74.61±99.6 |
70.83±115.7 |
0.874 |
||
|
Glasgow Blatchford score (mean±SD) |
9.22±4.6 |
11.61±3.2 |
3.85±1.9 |
<0.001 |
||
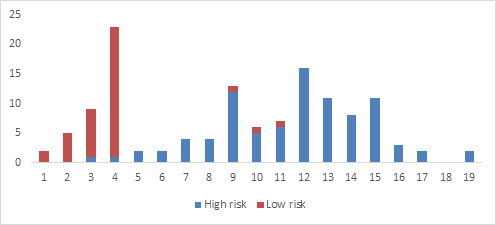
Figure 1: Number of high and low risk patients sorted according to Glasgow Blatchford score (n=130)
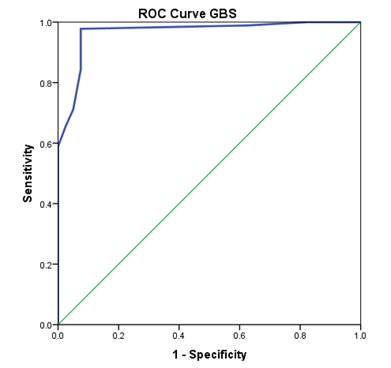
Figure 2: ROC curve for Glasgow Blatchford score to predict high risk patients with acute gastrointestinal bleeding
Table II: Sensitivity and specificity of various cut-offs for Glasgow Blatchford score
|
Cut off scores |
Sensitivity |
95% CI |
Specificity |
95% CI |
|
>1.0 |
100% |
98.5 – 100% |
5.0% |
3.3 - 7.2% |
|
>2.0 |
100% |
97.5 – 100% |
17.5% |
15.5 – 25.0% |
|
>3.0 |
98.0% |
95.0 – 100% |
37.5% |
35.0 – 45.5% |
|
>4.0 |
97.7% |
95.0 – 99.0% |
92.5% |
89.0 – 95.5% |
|
>5.0 |
95.5% |
93.5 – 96.0% |
92.5% |
88.0 – 96.5% |
Table III: Mean Glasgow Blatchford score for various risk factors
|
Risk Factors |
Mean Glasgow Blatchford score (mean±SD) |
P value |
|
|
Blood transfusion |
Yes (n=76) |
12.09 ± 3.1 |
<0.001 |
|
No (n=54) |
5.19 ± 3.2 |
||
|
Varices |
Grade I/II (n=39) |
9.38 ± 4.2 |
0.824 |
|
Grade III/IV (n=15) |
9.67 ± 3.8 |
||
|
Endoscopic findings |
Normal (n=8) |
4.00 ± 3.3 |
0.001 |
|
Abnormal (n=80) |
9.44 ± 4.3 |
||
|
ICU admission |
Yes |
12.16 ± 3.0 |
<0.001 |
|
No |
6.00 ± 3.8 |
||
|
Outcome |
Survived |
8.40 ± 4.5 |
<0.001 |
|
Died |
12.68 ± 3.5 |
||
DISCUSSION
Upper gastrointestinal bleeding is an important condition having life threatening consequences. Hence a good predictive score for evaluating the patients on time is vital. Our study population was aged around 60 years, which was similar to another study performed by Chattan et al.,16and Laursen et al.11 About half of patients were female (56.9%). Mortality rate in our study was 25 (27.8%), which was similar to another study performed by Chattan et al., 16 with mortality of 26. The reason for this is late presentation to emergency department by patients coming from far off areas from rural Pakistan. Poor financial conditions further add to the dilemma. Age and gender had no association with patient’s risk status or outcome. Low hemoglobin and platelet count were associated with high risk, similar to study performed by Hakan Tuncer et al., 6 (p value < 0.05), as low Hemoglobin indicated greater blood loss, and transfusion requirement is a risk factor for mortality as well as other high risk parameters in study. Higher ALT alkaline phosphatase, prothrombin and APTT as well as higher urea predicted high risk, similar to another study results 6. These parameters assess the severity of liver cirrhosis which was the main cause of bleeding in our study subjects.
Hypoalbuminemia also predicted poor outcome. As albumin is related to severity of liver disease, which may reflect its role as a predictive factor. In addition, albumin is a negative acute phase reactant which decreases in stress situations this may also be the reason for low albumin in high-risk group.
Mean GBS score of our population was 10.08±4.16. It was 12.68±3.5 in patients who died as compared to 8.4±4.5 in surviving patients (p <.001). Study published in Royal College Of Physicians 16 in 2018 had a mean GBS score of 5, however, their main finding on endoscopy was esophagitis, whereas in our study, majority patients had decompensated chronic liver disease, with variceal hemorrhage, which resulted in more severe derangement in clinical and lab parameters, hence resulting in a higher mean GBS score. Study by Hakan Tuncer et al., 6 had an overall mean GBS score of 13, which is slightly higher than ours. A reason for this difference can be higher number of patients suffering from other comorbidities like heart failure, malignancies, and higher use of anticoagulants and NSAIDS in their study.
A study12 was done on comparison of different scoring systems for the risk identification of upper gastrointestinal bleeding. It was concluded that GB scoring was more predictive in assessing the patients requiring endoscopy and mortality rates. However, it was concluded that all scoring systems were equally effective in predicting the mortality risks (<0.001). The lower scores predicted low risk. Scores greater than 7 showed more sensitivity and specificity in determining the risk ratios and patients requiring interventions. Alexandrio G et al.,7 documented that GB scoring was successful in predicting up to seven percent for 30-day mortality rate. The GB scoring predicted the outcomes of high-risk patients better than other scoring systems. However, it was further added in another research13 that 90-day mortality was predicted better by other scoring systems as compared to GB scoring. This highlights the fact that GB scoring can successfully predict outcomes in acute cases and is likely to help more in acute emergencies such as upper gastrointestinal bleeding.13
Another study5 reported that GB scoring as compared to another scoring system called clinical Rockall score was inadequately effective in predicating 30-day mortality and outcomes of upper gastrointestinal bleed.
A study by Islam MS et al.,6 documented that GB scoring predicts the low risk and high-risk individuals quite accurately. Similarly, another study from Korea, explaining the risk stratification of upper gastrointestinal bleed patients, concluded that GB scoring is effective in predicting the need of intervention and risk identification. This further decreases the hospital expenditure and burden of disease.8 However the need of intervention or the need of admission can be successfully predicted by GB scoring. AIMS65 has been considered more suitable for mortality-based14 prediction as compared to GB scoring. Whereas GB scoring can predict the need of interventions and need of admission more accurately.
Highlighting the accuracy and sensitivity of the GB scoring in predicting the need of intervention was supported by Duarte-Chang C et al.15 It was documented that GB scoring showed 98% sensitivity in predicting the need of endoscopy in patients with non-variceal bleed. They concluded that GB scoring has accurate diagnostic capacity to predict the need of intervention.
The accuracy of need of intervention was also studied in another research. The negative predictive value for excluding the need of intervention such as endoscopy was found to be hundred percent for score of up to one for GB scoring. The score of greater than three was able to predict the need of intervention and three patients with that score died later.16
Rout G, et al.,17 supported the same notion as described above for the predictiveness of all the scoring systems for hospital management and the chances of death and re-bleeding. However, their study focused more on non-variceal bleeds only. It was documented that GB scoring showed a negative predictive value of about 97% for non-variceal bleed outcomes and need of interventions.16 Similar was supported by the results documented by Gralnek IM, et al.18
Study performed by Renukaprasad et al., also had majority of patients with liver disease (43.2%), similar to our study, in which they compared different scoring systems in predicting clinical outcomes in upper gastrointestinal bleeding. They also found out that GB score was better at predicting the need for endoscopic intervention (AUROC 0.618, p 0.06), making it an accurate tool for timely management of critical patients.19
Study carried out in Switzerland also showed that GB score is more accurate in predicting the need for intervention, and that a GBS score of less than or equal to 1, can safely be managed as outpatient ,thus reducing unnecessary hospital admissions, thus supporting the findings in our study .20
A study by Franco et al., on cancer patients with upper gastrointestinal bleed, showed that GB better predicted blood transfusion requirement and accurately identified low risk group. Their study, however, showed that AIMS 65 was better in predicting ICU admissions and in hospital mortality. 21 Arya et al., also concluded similar findings in their study, that GB score was superior in predicting blood transfusion requirement and re bleeding risk.22
Boustany et al., used a GB score cut off of 2, which is lower than our threshold of 4, to predict low risk group, which can be managed as outpatient. The reason for their low threshold is that they excluded patients with known comorbidities and hemodynamic compromise, and our study population included such patients, hence the high score of 4 used as a cut off value.23
LIMITATION OF STUDY: The small number of patients and the single-center study were the limitations of this study. Our study population mainly consisted of patients with bleeding secondary to portal hypertension, so the results may be biased towards this subgroup. Furthermore, we analyzed portal hypertensive and non-portal hypertensive patients simultaneously due to the low number of later patients, although they are heterogeneous populations. Further studies analyzing the accuracy of the GB score in these groups separately are recommended to overcome these limitations.
CONCLUSION
In conclusion, this prospective cohort study at Fauji Foundation Hospital in Rawalpindi demonstrates the GB scoring system's robustness in assessing risk and outcomes for patients with UGIB. The GB score proves excellent in differentiating high and low-risk patients, predicting interventions, ICU admission, and mortality rates. Despite limitations such as a small sample size and single-center focus, the study contributes to the evidence supporting the efficacy of the GB scoring system in guiding timely and effective management of acute gastrointestinal bleeding. Further research in diverse patient populations is recommended to validate and extend these findings.
ACKNOWLEDGEMENTS
Special thanks to Gen Tasawwar (R), HoD Medicine, FFH Rawalpindi for his support.
REFERENCES
1. Mahajan P, Chandail VS. Etiological and Endoscopic Profile of Middle Aged and Elderly Patients with Upper Gastrointestinal Bleeding in a Tertiary Care Hospital in North India: A Retrospective Analysis. J Midlife Health 2017;8(3):137-41. https://doi.org/10.4103/jmh.jmh_86_17
2. Wilkins T, Wheeler B, Carpenter M. Upper Gastrointestinal Bleeding in Adults: Evaluation and Management. Am Fam Physician 2020;101(5):294-300. Erratum in: Am Fam Physician 2021 Jan 15;103(2):70
3. Aoki T, Hirata Y, Yamada A, Koike K. Initial management for acute lower gastrointestinal bleeding. World J Gastroenterol 2019;25(1):69-84. https://doi.org/10.3748/wjg.v25.i1.69
4. Costa-Moreira P, Macedo G. Risk Stratification in Upper Gastrointestinal Bleeding: A Measure of Safety and Efficiency in Emergency Care. GE Port J Gastroenterol 2021;28(4):231-3. https://doi.org/10.1159/000512091
5. Tuncer H, Yardan T, Akdemir HU, Ayyildiz T. Comparison of four scoring systems for risk stratification of upper gastrointestinal bleeding. Pak J Med Sci 2018;34(3):649-54. https://doi.org/10.12669/pjms.343.14956
6. Islam MS, Uddin MZ, Ali MS, Islam MN, Rahman MH, Robi IH, et al. Modified Blatchford Score for Risk Stratification in Adult Patient with Nonvariceal Upper Gastrointestinal Haemorrhage and Their Short Term Hospital Outcome. Mymensingh Med J 2017;26(3):490-97.
7. Alexandrino G, Carvalho R, Reis J. Comparison of the AIMS65 ore with Other Risk Stratification Scores in Upper Variceal and Nonvariceal Gastrointestinal Bleeding. Gut Liver 2018;12(1):111-3. https://doi.org/10.5009/gnl17380
8. Lee BE. Risk Stratification for Patients with Upper Gastrointestinal Bleeding. Korean J Helicobacter Up Gastrointest Res 2018;18(4):225-30 https://doi.org/10.7704/kjhugr.2018.18.4.225
9. Cai JX, Saltzman JR. Initial Assessment, Risk Stratification, and Early Management of Acute Nonvariceal Upper Gastrointestinal Hemorrhage. Gastrointest Endosc Clin N Am 2018;28(3):261-75. https://doi.org/10.1016/j.giec.2018.02.001
10. Robertson M, Ng J, Abu Shawish W, Swaine A, Skardoon G, Huynh A, et al. Risk stratification in acute variceal bleeding: Comparison of the AIMS65 score to established upper gastrointestinal bleeding and liver disease severity risk stratification scoring systems in predicting mortality and rebleeding. Dig Endosc 2020;32(5):761-8. https://doi.org/10.1111/den.13577
11. Laursen SB, Hansen JM, Schaffalitzky de Muckadell OB. The Glasgow Blatchford score is the most accurate assessment of patients with upper gastrointestinal hemorrhage. Clin Gastroenterol Hepatol 2012;10(10):1130-35. https://doi.org/10.1016/j.cgh.2012.06.022
12. Anchu AC, Mohsina S, Sureshkumar S, Mahalakshmy T, Kate V. External validation of scoring systems in risk stratification of upper gastrointestinal bleeding. Indian J Gastroenterol 2017;36(2):105-12 https://doi.org/10.1007/s12664-017-0740-x
13. Liu S, Zhang X, Walline JH, Yu X, Zhu H. Comparing the Performance of the ABC, AIMS65, GLASGOW BLATCHFORD SCORING, and pRS Scores in Predicting 90-day Mortality Or Rebleeding Among Emergency Department Patients with Acute Upper Gastrointestinal Bleeding: A Prospective Multicenter Study. J Transl Int Med 2021;9(2):114-22. https://doi.org/10.2478/jtim-2021-0026
14. Lu X, Zhang X, Chen H. Comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems for the prediction of the risk of in-hospital death among patients with upper gastrointestinal bleeding. Rev Esp Enferm Dig 2020;112(6):467-73 https://doi.org/10.17235/reed.2020.6496/2019
15. Duarte-Chang C, Beitia S, Adames E. Utilidad de la escala de Glasgow-Blatchford en pacientes con hemorragia digestiva alta no variceal, con alto y bajo riesgo de complicaciones atendidos en el Servicio de Urgencias del Hospital Santo Tomas, 2015-2016 [Glasgow-Blatchford usefulness in patients with non variceal upper gastrointestinal bleeding with low and high risk of complications seen at the Emergency Department of Santo Tomas Hospital, 2015-2016]. Rev Gastroenterol Peru 2019;39(2):105-10
16. Chatten K, Purssell H, Banerjee AK, Soteriadou S, Ang Y. Glasgow Blatchford Score and risk stratifications in acute upper gastrointestinal bleed: can we extend this to 2 for urgent outpatient management? Clin Med 2018;18(2):118-22 https://doi.org/10.7861/clinmedicine.18-2-118
17. Rout G, Sharma S, Gunjan D, Kedia S, Nayak B, Shalimar. Comparison of various prognostic scores in variceal and non-variceal upper gastrointestinal bleeding: A prospective cohort study. Indian J Gastroenterol 2019;38(2):158-66 https://doi.org/10.1007/s12664-018-0928-8
18. Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy 2021;53(3):300-32 https://doi.org/10.1055/a-1369-5274
19. Renukaprasad AK, Narayanaswamy S, R V. A Comparative Analysis of Risk Scoring Systems in Predicting Clinical Outcomes in Upper Gastrointestinal Bleed. Cureus 2022 Jul 8; https://doi.org/10.7759/cureus.26669
20. Rivieri S, Carron PN, Schoepfer A, Ageron FX. External validation and comparison of the Glasgow-Blatchford score, modified Glasgow-Blatchford score, Rockall score and AIMS65 score in patients with upper gastrointestinal bleeding: a cross-sectional observational study in Western Switzerland. Eur J Emergen Med 2022;30(1):32-9. https://doi.org/10.1097/mej.0000000000000983
21. Franco MC, Jang S, Martins B da C, Stevens T, Jairath V, Lopez R, et al. Risk Stratification in Cancer Patients with Acute Upper Gastrointestinal Bleeding: Comparison of Glasgow-Blatchford, Rockall and AIMS65, and Development of a New Scoring System. Clin Endoscopy 2022;55(2):240-7. https://doi.org/10.5946/ce.2021.115
22. Arya P, Thulaseedharan NK, Raj R, Dileep Unnikrishnan, Jacob A. AIMS65, Glasgow-Blatchford bleeding score and modified Glasgow-Blatchford bleeding score in predicting outcomes of upper gastrointestinal bleeding: An accuracy and calibration study. Indian J Gastroenterol 2023;42(4):496-504 https://doi.org/10.1007/s12664-023-01387-z
23. Boustany A, Alali A, Almadi MA, Martel M, Barkun AN. Pre-Endoscopic Scores Predicting Low-Risk Patients with Upper Gastrointestinal Bleeding: A Systematic Review and Meta-Analysis. J Clin Med 2023;12(16):5194. https://doi.org/10.3390/jcm12165194
|
Following authors have made substantial contributions to the manuscript as under:
FK: Study design, acquisition of data, drafting the manuscript, approval of the final version to be published NY & SP: Concept and study design, critical review, approval of the final version to be published HQ & MIK: Analysis and interpretation of data, drafting the manuscript, approval of the final version to be published Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest
GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |