![]() https://doi.org/10.35845/kmuj.2023.22728 ORIGINAL ARTICLE
https://doi.org/10.35845/kmuj.2023.22728 ORIGINAL ARTICLE
Gender differences in plasma levels of cardiovascular risk markers and severity indices in Saudi patients with angiographic evidence of coronary artery disease
Syed Shahid Habib 1 ![]() ,
Thamir Al-khlaiwi 1, Abdulrahman Alhowikan1, Zohair Al
Aseri 2, Syed Mohammad Habib3, Huthayfah Al-khliwi1,
Ahmad Omair4
,
Thamir Al-khlaiwi 1, Abdulrahman Alhowikan1, Zohair Al
Aseri 2, Syed Mohammad Habib3, Huthayfah Al-khliwi1,
Ahmad Omair4
|
1: Department of Physiology, College of Medicine and King Khalid University Hospital, Riyadh, Kingdom of Saudi Arabia. 2: Departments of Emergency Medicine and Critical Care, College of Medicine, King Saud University, Riyadh, Saudi Arabia. 3: College of Medicine, Sulaiman Al Rajhi University, Al Bukayriah, Saudi Arabia. 4: College of Science & Health Professions, King Saud bin Abdulaziz University for Health Sciences & King Abdullah International Medical Research Center, Riyadh, Kingdom of Saudi Arabia. Email Contact #: +966-508942522; +966-1-4671616 Date Submitted: April 07, 2022 Date Revised: April 04, 2023 Date Accepted: April 14, 2023 |
|
THIS ARTICLE MAY BE CITED AS: Habib SS, Al-khlaiwi T, Alhowikan A, Aseri ZA, Habib SM, Al-khliwi H, et al. Gender differences in plasma levels of cardiovascular risk markers and severity indices in Saudi patients with angiographic evidence of coronary artery disease. Khyber Med Univ J 2023;15(2):71-7. https://doi.org/10.35845/kmuj.2023.22728
|
ABSTRACT
OBJECTIVE: To evaluate gender difference in non-traditional cardiovascular risk factors among Saudi patients with angiographically defined coronary artery disease (CAD).
METHODS: We recruited 144 (male=105, female=39) patients with CAD who underwent coronary angiography. Laboratory analysis of total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), lipoprotein(a) [Lp(a)], and high sensitivity C-reactive protein (hs-CRP) were performed along with echocardiographic evaluation.
RESULTS: Systolic blood pressure (SBP) was significantly higher in females (138.65±18.67 vs 129.59±21.03 mmHg; p=0.0363). Although no significant difference was observed in TC (4.30±1.36 vs 4.67±1.07 mmol/L), however significantly higher levels of both HDL and LDL were seen in females (0.86±0.17 vs 0.70±0.23 mmol/L; p=0.0592 and 3.50±0.67 vs 2.72±1.13; p=0.0466 respectively). There were no significant differences in TG, Lp(a) and hs-CRP, however females had comparatively higher Lp(a) (30.43±28.89 vs 24.02±23.42 mg/dl) and hs-CRP (1.14±1.46 vs 0.95±1.25 mg/L). On angiography, greater extent of stenosis was observed in left anterior descending and right coronary arteries among males, while the left circumflex artery was affected more in the female patients without statistical significance (p=0.0797; 0.3890 and 0.5032 respectively). Gensini Score in males & females was 60.46±46.17 and 74.39±39.52 respectively (p=0.3013). Significantly narrower aortic root diameter (ARD) was observed among females (27.30±6.66 vs 29.73±3.40 mm; p=0.0293).
CONCLUSION: Female patients have greater risk of cardiovascular diseases due to higher SBP and LDL levels which could lead to a greater Gensini score when compared to male patients. In addition, narrow ARD among females could also be a contributing factor to CAD.
KEYWORDS: Coronary Artery Disease (MeSH); Angiography (MeSH); Gensini Score (Non-MeSH); Risk Factors (MeSH); Sex Characteristics (MeSH); Cholesterol (MeSH);Triglycerides (MeSH); Lipoproteins (MeSH); Lipoproteins, HDL (MeSH); Lipoproteins, LDL (MeSH); Lipoprotein(a) (MeSH).
INTRODUCTION
Mortality among females due to cardiovascular diseases is higher than males due to breast and ovarian cancers combined.1 Two groups of risk factors exist, modifiable which can be controlled by lifestyle intervention and medications while non-modifiable which cannot be altered, such as gender and genetic makeup.2
In post-menopausal period, due to the decrease in levels of estrogen, incidence of coronary artery disease (CAD)3 and hyperlipidemia are increased.4
Usually, the first myocardial infarction (MI) in men preceded the women by approximately ten years even though the increased incidence of CAD is reported with advancing age in both sexes with different risk factors.5
Because of lower incidence of CAD in younger women compared to age matched men, a false popular misconception that it is a disease of men and is relatively rare in women has become a fact and cannot be negotiated. Women have less risk factors of cardiovascular diseases than men at any given age.6 Usually, females have higher level of HDL which play a protective role, compared to males.7 Another study has shown women have higher levels of HDL than men and lower TGs.8
This false misconception was led by many trials that have been done in white, middle-aged men in which women were not well-represented.9 Additionally and also due to treatment bias, females with CAD have been reported to have worse outcome compared to males. Multiple complicating factors such as diabetes, and hypertension might also lead to poor outcome neutralizing the benefit imparted by female hormones. Treatment trials sometimes cannot be taken into consideration due to gender differences.10
Most of the studies related to cardiovascular risk factors have been performed on mostly male patient sample and the data has been extrapolated to females.11 Subsequently, the influence of estrogen on lipid profile is sometime ignored which might lead to unexpected outcome when applied to the female patients.1,3 In reality, the incidence of cardiovascular disease might be lower among younger women compared to young men up to the age of 65 and the risk becomes equal for both sexes afterwards.11 Therefore, gender difference could interfere with all stages of CAD, beginning from risk factors and ending with therapeutic modalities.7
Lipoprotein(a) [Lp(a)] has been reported to be an independent risk factor for CAD and significantly associated with increased CAD risk in pre- and postmenopausal women.12,13
The difference in high sensitivity C-reactive protein (hs-CRP) between males and females have been studied worldwide in various diseases and some have mentioned significant gender differences.14 Whether there is a gender difference in hs-CRP in CAD or not is a question that needs to be answered. Whether there are gender differences in CAD biomarkers and risk factors between male and female Saudi population needs to be assessed. There is scanty and controversial data on the gender differences in cardiovascular risk factors in patients with CAD especially for non-traditional risk factors. Therefore, we aimed to evaluate gender differences in plasma lipids [total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL)], Lp(a), and hs-CRP levels, echocardiographic findings, blood pressure, and extent of CAD among male and female patients with angiographic evidence of CAD.
METHODS
This comparative cross-sectional study was conducted at the Department of Physiology and King Fahad Cardiac Center, of King Saud University Medical City, King Saud University, Riyadh, Kingdom of Saudi Arabia from June 2018 to December 2019 and has been approved by the Research Ethics Committee of College of Medicine Research Center (CMRC) Ref No: 26-60. A total of 145 patients (males = 105, females = 39) were enrolled by convenience sampling technique after signing the consent form. Literature search and power calculation for similar studies revealed a minimum 80% predictive power at 95% confidence interval an appropriate sample size of about 140 subjects.15 The information obtained for each participant included personal and clinical characteristics, laboratory results, extent of ischemic heart disease (IHD) on coronary angiography, and the history of medication. The patients were divided into two groups based on gender and compared for different variables as mentioned earlier. Body mass index (BMI) was also calculated and compared between two groups as obesity is a risk factor for CAD. Lipid profile and lipoproteins were used for risk evaluation & Gensini score was calculated for severity. Comorbidities like diabetes, hypertension and smoking status were also evaluated and compared between the two groups.
Patient sample
Inclusion criteria:
Adult patients of any gender and age with evidence of CAD and underwent coronary angiography.
Exclusion criteria:
Any conditions which cause metabolic dysregulation and have significant effects on analytes studied were excluded for example acute or chronic renal failure, nephrotic syndrome, thyroid problems, acute and chronic infections (tuberculosis, chronic hepatitis etc), recent stroke, diabetic acute states like diabetic ketoacidosis (DKA) and non-ketotic hyperosmolar coma (NKHC). In addition, women taking oral contraceptives and patients under steroid therapy were also excluded from the study. Patients with history of any acute coronary event like MI or unstable angina in last two months were excluded.
Based on inclusion and exclusion criteria, 144 (105 male and 39 female) patients were included in the study. Overnight fasting (12 hours) blood samples were collected, serum was separated and were stored at – 70 °C.
Laboratory parameters
Lipid profile:
Venous blood samples were obtained after 10-12 hours of overnight fasting and analysis was made for TC, TG, LDL, and HDL using autoanalyzer and kits from Dimension-USA that uses photoelectric colorimetric principle. Lp(a) was analyzed by turbidimetric immunoassay using Hitachi 911, manufactured by ROCHE diagnostics, USA, with quantex Lp(a) kits. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) developed the reference material SRM2B which is also approved by the WHO Expert Committee on Biological Standardization as the first WHO/IFCC International Reference Reagent for Lp(a) Immunoassay. The lower limit of detection (LOD) was 0.4 mg/dl. hs-CRP assay was performed using turbidimetric assay method using Quantex hs-CRP kits supplied by BIOKIT Spain and Hitachi 911 autoanalyzer manufactured by ROCHE diagnostics, USA. The kit detected hs-CRP in ranges of 0.10 mg/L to 20.0 mg/L. We followed American Heart Association criteria for measurement, evaluation, and expression of hs-CRP.
Coronary Angiography
Based on coronary vessel involvement, patients were divided into three groups: single, double and triple vessel disease. All participants had undergone left ventriculography and selective coronary angiography using the right femoral artery approach. Right and left oblique views with cranial and caudal positions were used to visualize and image coronaries. At least 50% stenosis in a major coronary segment was regarded as significant stenosis. Gensini scoring system was used to determine the CAD severity by getting of percentage luminal narrowing and localization.16 Left main coronary artery, left anterior descending artery (LAD), left circumflex (LCx) and right coronary arteries (RCA) were assessed. Multiple lesions in the same vessel were considered as single vessel disease.
Echocardiography
Parameters recorded by Echocardiography were: (ARD), aortic root diameter (LAD), left atrial dimension, (LVIDd), left ventricular internal diameter in diastole (LVIDs), left ventricular internal diameter in systole: left ventricular posterior wall thickness (PW) , interventricular septal thickness (IVS) and ejection fraction (EF).
Statistical Analysis
The data was analyzed by Statistical Package for Social Sciences (SPSS version 21.0, Chicago, USA). Descriptive parameters and lipid profile of the patients were calculated as mean & median ± SD (Standard Deviation), SEM (Standard Error of Mean) for continuous variables, and as percentages for categorical variables. The tests applied for statistical analysis were Student’s t test for continuous variables and Chi square for proportions. A p value of £ 0.05 was considered as statistically significant. The relative percentage distribution of individuals in different groups with desirable and high-risk levels of Lp(a) was determined.
RESULTS
The mean age of our sample was 54.82±12.37 years with a range between 23 and 80 years. The mean age of the male and female patients was 53.94±12 and 57.35±14.46 years respectively. The age range between male and female patients was not significantly different (26 to 80 and 23 to 80 years respectively). There was no statistically significant difference between age of the two genders (p = 0.1795).
Table I shows comparison of demographic and clinical characteristics of male and female patients with CAD. In comparison with the male patients, the systolic blood pressure among females was observed to be significantly higher (138.65±18.67 vs 129.59±21.03 mmHg; p = 0.0363). The diastolic blood pressure was also higher among females (80.23±13.46 vs 75.68± 14.94), however this difference was not found to be statistically significant (p = 0.1384). Females were also found to have significantly less height compared to the males (154.00±8.55 vs 165.11±7.98; p = 0.0000). No statistically significant difference was observed for all the remaining demographic and clinical parameters.
Table I: Comparison of demographic and clinical characteristics among male and female patients (Mean ± SD and counts with percentages)
|
Variables |
Males (n = 105) |
Females (n =39) |
p-value |
|
Age (years) |
53.94 ±12.00 |
57.35 ± 14.46 |
0.1795 |
|
Height (cm) |
165.11 ± 7.98 |
154.00 ± 8.55 |
0.0001 |
|
Weight (kg) |
76.95 ±14.30 |
72.11 ± 17.69 |
0.2417 |
|
BMI (kg/m2) |
27.12 ± 7.71 |
23.81 ± 13.24 |
0.1596 |
|
Pulse (beats per minute) |
82.62 ± 15.62 |
86.81 ± 15.99 |
0.2057 |
|
Respiratory Rate (per minute) |
20.49 ± 2.17 |
20.81 ± 3.68 |
0.5636 |
|
Temp (degree Celsius) |
36.52 ± 0.55 |
36.59 ± 0.62 |
0.6003 |
|
Standing Systolic BP (mmHg) |
0.0363 |
||
|
Standing Diastolic BP (mmHg) |
0.1384 |
||
|
Diabetes Mellitus n (%) |
61 (58.1%) |
26 (66.7%) |
0.3499* |
|
Hypertension n (%) |
54 (51.4%) |
22 (56.4%) |
0.1019 * |
|
Dyslipidemia n (%) |
23 (21.9%) |
11 (28.2%) |
0.4288 * |
|
Current Smokers n (%) |
18 (17.1%) |
0 (0%) |
NA* |
|
Ex-Smokers n (%) |
27 (25.7%) |
0 (0%) |
NA* |
The differences were analyzed by Student’s t test; *Chis-square test; NA: not applicable
Table II showed difference in laboratory parameters between males and females. No statistically significant difference in TC was observed between male and female patients (4.30±1.36 vs 4.67±1.07; p = 0.1795), however it showed a significantly higher level of HDL among females compared to males (0.86 ± 0.17 vs 0.70 ± 0.23; p = 0.0592). At the same time, LDL was also significantly higher among females (3.50±0.67 vs 2.72±1.13; p = 0.0466). TGs were lower in female patients with no significant difference (1.46±0.79 vs 1.85±1.06; p = 0.1380). Lp(a) and hs-CRP were higher among females compared to males (30.43±28.89 vs 24.02±23.42 and 1.14±1.46 vs 0.95±1.25 respectively), however the difference was statistically not significant (p = 0.1867 and p = 0.4645 respectively).
Table II: Comparison of laboratory parameters among male and female patients
(Mean ± SD)
|
Laboratory Parameters |
Males (n = 105) |
Females (n = 39) |
p-value |
|
TC (mmol/L) |
4.67 ± 1.07 |
0.2690 |
|
|
HDL (mmol/L) |
0.0592 |
||
|
2.72 ± 1.13 |
3.50 ± 0.67 |
0.0466 |
|
|
TG (mmol/L) |
0.1380 |
||
|
Lp(a) mg/dl |
24.02 ± 23.42 |
30.43 ± 28.89 |
0.1867 |
|
hs-CRP mg/L |
0.95 ± 1.25 |
1.14 ± 1.46 |
0.4645 |
*The differences were analyzed by Student’s t test. TC: total cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; TG: triglycerides; Lp(a): lipoprotein A; hs-CRP: highly sensitive CRP.
Table III showed that extent (percentage) of stenosis among male and female patients. LAD and RCA involvement was more in male patients compared to the females (85.34 %±16.72 vs 73.75%±21.17, and 80.80% ± 21.21 vs 72.86% ± 29.84 respectively). Although difference in involvement of RCA was not significant (p = 0.389), a trend towards significance was observed for difference in the extent of involvement of LAD between two genders (p = 0.0797). On the other hand, the LCx was found to be more affected in the female patients though the difference was insignificant (85.00%±21.79 vs 78.80±23.03; p = 0.50). The severity assessment using Gensini Score revealed higher mean score in female patients (74.39±39.52) compared to the male patients (60.46±46.17) with an insignificant p value of 0.3013.
Table III: Comparison of extent of stenosis (%) in coronary arteries and Gensini score, among male and female patients (Mean ± SD)
|
Stenosed artery |
Males (n = 105) |
Females (n = 39) |
p-value |
|
LAD |
85.34 ± 16.72 |
73.75 ± 21.17 |
0.0797 |
|
LCx |
78.80 ± 23.03 |
85.00 ± 21.79 |
0.5076 |
|
RCA |
80.80 ± 21.21 |
72.86 ± 29.84 |
0.3890 |
|
60.46 ± 46.17 |
74.39 ± 39.52 |
0.3013 |
*The differences were analyzed by Student’s t test. LAD: left anterior descending; LCx: left circumflex; RCA: right coronary arteries.
Assessment of echocardiographic parameters (Table 4) revealed significantly narrower aortic root diameter (ARD) among female patients compared to the males (27.30±6.66 vs 29.73±3.40; p=0.0293). No statistically significant difference was observed for other parameters between the two genders (Table IV).
Table IV: Comparison of echocardiographic parameters among male and female patients
(Mean ± SD)
|
Parameters |
Males (n =105) |
Females (n =39) |
p-value |
|
ARD (mm) |
29.73 ± 3.40 |
27.30 ± 6.66 |
0.0293 |
|
LAD (mm) |
38.03 ± 5.23 |
38.83 ± 4.79 |
0.5257 |
|
LVIDd (mm) |
52.90 ± 7.90 |
50.68 ± 5.31 |
0.2251 |
|
LVIDs (mm) |
36.26 ± 9.49 |
33.23 ± 5.40 |
0.1608 |
|
PW (mm) |
10.27 ± 1.80 |
9.59 ± 1.30 |
0.1067 |
|
IVS (mm) |
10.69 ± 2.13 |
10.27 ± 1.75 |
0.4084 |
|
EF (%) |
40.42 ± 13.25 |
40.71 ± 11.32 |
0.9279 |
*The differences were analyzed by Student’s t test. ARD: aortic root diameter; LAD: left atrial dimension; LVIDd: left ventricular internal diameter in diastole; LVIDs: left ventricular internal diameter in systole; PW: left ventricular posterior wall thickness; IVS: interventricular septal thickness; and EF: ejection fraction.
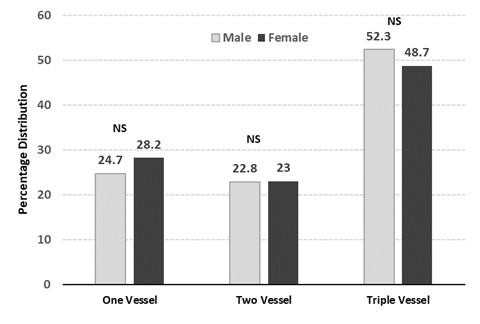
Figure 1: Gender comparison of percentage distribution in vessel score; Proportions were compared by Chi Square test: NS: non-significant
DISCUSSION
There is a clear mismatch in diagnosis of CAD among males and females with males accounting for most of the diagnosed cases, yet the incidence increases with age in both sexes. What being even more alarming is an increase in CVD associated mortality among younger women,17 owing partly to the misconception that it is mainly a disease of men and females are protected with effects of estrogen in their younger reproductive age group. This fact warrants the importance of evaluating the gender associated risk by assessing the differences in various cardiovascular risk factors among both sexes. This study evaluated the gender difference in these risk factors among Saudi CAD cases revealing the deranged lipid profile and hypertension as significant risk factors.
Among our CAD cohort, females were comparatively older compared to males (mean age: 57.35 vs 53.39 years). Older age group itself is a risk factor for CAD in both sexes because of marked atherosclerosis, but its importance cannot be neglected specifically in women. Older age group is associated with various other risk factors among females which are attributed to decreasing level of estrogens in perimenopausal and postmenopausal period. The mean age of our cohort indicates that most of our cases were postmenopausal. High blood pressure is a major risk factor for the development of CAD attributed mainly to atherosclerosis with increasing age, but with difference in the incidence among the two genders. It is well known that blood pressure is higher in males compared to females up to 50 years of age, but afterwards the incidence in women exceeds that in men. 18 We report an increase in incidence of hypertension among females (56.4% vs 51.4%) and a statistically significant higher systolic blood pressure compared to the males, which highlights the importance of hypertension as a major risk factor for CAD among women above 50 years of age. Therefore, we dispute the previous misconception that elevated systolic blood pressure in the elderly to be considered normal and harmless.2 This increase in systolic blood pressure is thought to be because of the effect of menopause on the arterial compliance, as well as gender difference in various mechanisms responsible for controlling blood pressure including the renin-angiotensin system. Our findings are in line with what has been reported by Staessen et al. who followed up the blood pressure of 315 women for a median of 5.2 years and revealed a 4–5 mmHg significantly higher systolic blood pressure among postmenopausal compared to the premenopausal women who had no significant change in systolic blood pressure in follow-up.19
Although studies have reported a significantly increased risk of CAD among patients with increased BMI,20 we did not find a statistically significant gender difference in BMI among our CAD cases though males had a relatively higher BMI compared to females.
The importance of deranged lipid profile (higher TC, LDL, Lp(a), TG and lower HDL) in accelerated atherosclerosis and as an independent risk factor for CAD or indirectly by causing
Hypertension cannot be undermined. Due to the presence of female sex hormones, women significantly differ from men in lipid profile and apolipoproteins, regardless of age and menopausal status. This has been identified in large epidemiological studies and hence might explain the difference in the incidence of CAD between different sexes. We also observed statistically significant gender differences in lipid profile among our CAD cohort. TC was higher among females though not statistically significant, but the LDL was significantly higher among females. These findings are in line with the findings of Nikkila et al. who reported a higher TC among elderly females.21 Our findings are further strengthened by the Framingham Study, that revealed high in serum cholesterol levels in women between the premenopausal and postmenopausal stages, with the rise taking place within a short time of the onset of the menopause. 13,22As the mean age of our female CAD cases was (57.35 years), we also attribute these higher TC and LDL to the effect of hormonal changes during post menopause, nevertheless, not all studies have been able to confirm this relationship such as the study in Pima Indian women, there was no association between the menopausal status and high cholesterol,23 may be due to their healthy diet, which is generally lower in cholesterol compared to diet of White population. Therefore, lifestyle and environmental factors may play an additional and important role to increase cholesterol levels observed in older White women.
Another interesting finding in our study was a significantly higher HDL levels among female cases, which is usually thought to be protective against CAD due to its protective influence on inflammation, oxidation, and glucose metabolism.24 Literature also shows that women have comparatively higher production rates of apoA-I, which is a major HDL apoprotein, and that production of apoA-I and LpA-I can be increased with estrogen administration. Our finding of a significantly higher HDL levels among elderly females and lower HDL levels among comparatively younger males with CAD are in line with a previous report of elderly women younger men having lower HDL cholesterol21 and women having higher levels of HDL.8 This contradictory yet an interesting finding in our study of significantly higher levels of both LDL and HDL in females compared to the males, highlights the importance of role of LDL as a risk factor among elderly women, which according to our findings override and suppress the protective influence of HDL.
Similarly, we have reported in this study, an insignificant increase in Lp(a) and hs-CRP among females compared to the males. Lp(a) has been reported to be an independent risk factor for premature CVD irrespective of the higher LDL levels, predominantly due to its atherosclerotic, prothrombotic and anti-fibrinolytic role.25 There exists a controversy in literature, regarding the association of female gender and female sex hormones with raised Lp(a) levels. In a study by Lip et al. post hysterectomy and bilateral oophorectomy the plasma levels of Lp(a) were significantly increased compared to the levels before.13,26 Whereas Framingham Offspring study, showed non-significant association between menopause status and Lp(a) levels.27 On the other hand, our finding of an increased level of hs-CRP among females are in line with findings of Habib et al. who reported a significantly higher hs-CRP levels in patients with severe CAD compared to healthy controls,28 as well as Qasim et al. who reported an association of CRP with calcification of coronary arteries and ultimately CAD among females.29
Compared to the males, we also observed lower levels of TG among female cases. Though this difference was also statistically insignificant but is strengthened by the findings of Hafe et al. who had shown that women have lower levels of TG compared to men.8
Last but not the least, the association of diabetes with CAD cannot be neglected. In our study, compared to the males a significantly higher percentage of female CAD cases had diabetes (66.7% vs 51.4%). Studies have classified diabetic women to be at the same risk of CAD as men of the same age.2, 30 Much of this increased risk of CAD in diabetics can be attributed to its influence on blood pressure, glucose tolerance, and lipid levels (metabolic syndrome). Therefore, the presence of diabetes seems to negate any cardio protection that can be attributed to the female gender at any age group.
Our echocardiographic findings revealed a statistically significant narrow aortic root diameter among female cases compared to the males. This could be another contributing factor to the incidence of CAD among females along with the deranged lipid profile and glucose tolerance. The narrow aortic root may contribute to CAD by causing turbulent blood flow as in hypertension. Our findings are strengthened by Bahlmann et al. involving 1560 patients with aortic stenosis, who reported a strong association between narrow aortic root and an increased risk of CAD and mortality, even in the absence of known risk factors.31 We suggest that there exists a strong corelation between the narrow aortic root and atherosclerosis.
The strengths of this study include a reasonable overall sample size, assessment of an extensive clinical, laboratory and echocardiographic risk factors among CAD cased of both genders. Also, the inclusion based on presence of CAD was confirmed by coronary angiography. A relatively a smaller number of female cases is a limitation of this study.
CONCLUSION AND RECOMMENDATIONS
Female patients have greater risk of cardiovascular diseases due to higher systolic blood pressure and LDL levels which could lead to a greater Gensini score when compared to male patients. In addition, narrow ARD among females could also be a contributing factor to CAD. Gender specific diagnostic and treatment plans should be designed to facilitate early diagnosis and preventing mortality among females. More research studies are needed to confirm these findings. Better knowing of these risk factors and their gender differences can reduce mortality and morbidity due to cardiovascular diseases as well as cerebrovascular and peripheral vascular disease.
REFERENCES
1. Palmisano B, Zhu L, Eckel R, Stafford J. Sex differences in lipid and lipoprotein metabolism. Mol Metab 2018;15:45-55. https://doi.org/10.1016/j.molmet.2018.05.008
2. Hajar R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017;18(3):109-14. https://doi.org/10.4103/heartviews. heartviews_106_17
3. Palmisano B, Zhu L, Stafford J. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv Exp Med Biol 2017:1043:227-56. https://doi.org/10.1007/978-3-319-70178-3_12
4. Blumel JE, Castelo-Branco C, Rocangliolo ME, Bifa L, Tacla X, Mamani L. Changes in body mass index around menopause: a population study of Chilean woman. Menopause 2001;8(4):239-44. https://doi.org/10.1097/00042192-200107000-00004
5. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119(3):e21-181. https://doi.org/10.1161/CIRCULATIONAHA.108.1912616
6. Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011. Circulation 2015;132(11):997. https://doi.org/10.1161/CIRCULATIONAHA.115.015293
7. Legato MJ. Dyslipidemia, gender, and the role of high-density lipoprotein cholesterol: implications for therapy. Am J Cardiol 2000;86(12A):15L-18L. https://doi.org/10.1016/s0002-9149(00)01463-6
8. Hafe P. Gender differences in lipid profile and therapy. Rev Port Cardiol 2019;38(8):571-2. https://doi.org/10.1016/j.repc.2019.09.003
9. Khaw KT. Where are the women in studies of coronary heart disease? Br Med J 1993;306:1145-6. https://doi.org/10.1136/bmj.306.6886.1145
10. Considering gender in prescribing statins: what do physicians need to know?, Clin Lipidol 2015;10(6):499-512. https://doi.org/10.2217/clp.15.39
11. Fowler PB. Cardiovascular risk in women: the cardiologist's perspective. J Assoc Physicians 2000;93(6):387-8. https://doi.org/10.1093/qjmed/93.6.387-a
12. Habib SS. Lipoprotein(a). The bad cholesterol. Saudi Med J 2004;25(4):429-33.
13. Aljawini N, Aldakhil LO, Habib SS. High-Risk Lipoprotein(a) Levels in Saudi Women and Its Relationship to Menopause and Adiposity. Nutrients 2023;15(3):693. https://doi.org/10.3390/nu15030693
14. Hong GB, Gao PC, Chen YY, Xia Y, Ke XS, Shao XF et al. High-Sensitivity C-Reactive Protein Leads to Increased Incident Metabolic Syndrome in Women but Not in Men: A Five-Year Follow-Up Study in a Chinese Population. Diabetes Metab Syndr Obes 2020;13:581-90. https://doi.org/10.2147/DMSO.S241774
15. Málek F, Dvořák J, Skalníková V, Mates M, Kmoníček P, Vávrová Z, Neužil P. Correlation of lipoprotein(a) with the extent of coronary artery disease in patients with established coronary atherosclerosis: gender differences. Eur J Prev Cardiol 2015;22(5):603-5. https://doi.org/10.1177/2047487314527849
16. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51(3):606. https://doi.org/10.1016/s0002-9149(83)80105-2
17. Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol 2007;50:2128-32. https://doi.org/10.1016/j.jacc.2007.05.056
18. Reckelhoff JF. Gender differences in hypertension. Curr Opin Nephrol Hypertens 2018;27(3):176-81. https://doi.org/10.1097/MNH.0000000000000404
19. Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Human Hypertens 1997;11:507-14. https://doi.org/10.1038/sj.jhh.1000476
20. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol 2018;3(4):280-87. https://doi.org/10.1001/jamacardio.2018.0022
21. Nikkila M, Pitkajarvi T, Koivula T, Lehtomaki E, Jokela H. Age-related changes and sex differences in serum lipid concentrations in health-screening subjects. Cardiovasc Risk Factors 1991;1:204-10.
22. Hjortland MC, McNamara PM, Kannel WB. Some atherogenic concomitants of menopause: the Framingham Study. Am J Epidemiol 1976;103:304-11. https://doi.org/10.1093/oxfordjournals.aje.a112228
23. Hamman RF, Bennett PH, Miller M. The effect of menopause on serum cholesterol in American Pima Indian women. Am J Epidemiol 1975;102:164-9. https://doi.org/10.1093/oxfordjournals.aje.a112143
24. Nicholls SJ, Nelson AJ. HDL and cardiovascular disease. Pathology 2019;51(2):142-7. https://doi.org/10.1016/j.pathol.2018.10.017
25. Habib SS, Abdel-Gader AM, Kurdi MI, Al-Aseri Z, Soliman MM. Lipoprotein(a) is a feature of the presence, diffuseness, and severity of coronary artery disease in Saudi population. Saudi Med J 2009;30(3):346-52.
26. Lip GYH, Blann AD, Jones AF, Beevers DG. Effects of hormone-replacement therapy on hemostatic factors, lipid factors, and endothelial function in women undergoing surgical menopause: Implications for prevention of atherosclerosis. Am Heart J 1997;134:764-71. https://doi.org/10.1016/s0002-8703(97)70062-0
27. Jenner JL, Ordovas JM, Lamon-Fava S, Schaefer MM, Wilson P, Castelli WP etal. Effects of age, sex, and menopausal status on plasma lipoprotein(a) levels: the Framingham Offspring Study. Circulation 1993;87:1135–41. https://doi.org/10.1161/01.cir.87.4.1135
28. Habib SS, A Al Masri A. Relationship of high sensitivity C-reactive protein with presence and severity of coronary artery disease. Pak J Med Sci 2013;29(6):1425-9. https://doi.org/10.12669/pjms.296.330228
29. Qasim AN, Budharaju V, Mehta NN, St Clair C, Farouk S, Braunstein S et al. Gender differences in the association of C-reactive protein with coronary artery calcium in type-2 diabetes. Clin Endocrinol (Oxf) 2011;74(1):44-50. https://doi.org/10.1111/j.1365-2265.2010.03879.x
30. Habib SS, Aslam M, Hameed W. Gender differences in lipids and lipoprotein (a) profiles in healthy individuals and patients with type 2 diabetes mellitus. Pak J Physiol 2005; 1(1-2).
31. Bahlmann E, Cramariuc D, Minners J, Lønnebakken MT, Ray S, Gohlke-Baerwolf C, et al. Small aortic root in aortic valve stenosis: clinical characteristics and prognostic implications. Eur Heart J Cardiovasc Imaging 2017;18(4):404-12. https://doi.org/10.1093/ehjci/jew159. Erratum in: Eur Heart J Cardiovasc Imaging 2017;18(5):611. https://doi.org/10.1093/ehjci/jew159
|
AUTHORS' CONTRIBUTIONS Following authors have made substantial contributions to the manuscript as under:
SSH, TAl-K & AO: Concept and study design, acquisition, analysis and interpretation of data, drafting the manuscript, approval of the final version to be published
AA: Acquisition, analysis and interpretation of data, drafting the manuscript, approval of the final version to be published
ZAlA, HA & SMH: Acquisition, analysis and interpretation of data, critical review, approval of the final version to be published
Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest
GRANT SUPPORT AND FINANCIAL DISCLOSURE The project was supported by grant from Deanship of Scientific Research (Grant Number: RGP-1438-048) King Saud University, Riyadh, Saudi Arabia. |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |