![]() https://doi.org/10.35845/kmuj.2023.22498 SYSTEMATIC REVIEW AND META-ANALYSIS
https://doi.org/10.35845/kmuj.2023.22498 SYSTEMATIC REVIEW AND META-ANALYSIS
Diagnostic and prognostic role of the SARS-COV-2-specific IgA antibody: an updated
systematic review and meta-analysis
Hamda Shazam1, Yasir Butt2
![]()
|
1: Department of Oral Pathology, College of Dentistry, Ziauddin University, Karachi, Pakistan 2: Pakistan Kidney and Liver Institute and Research Centre (PKLI&RC), Lahore, Pakistan
Email Contact #: +92-332-4123101 Date Submitted:January28, 2022 Date Revised:February04, 2023 Date Accepted:February 08, 2023 |
|
THIS ARTICLE MAY BE CITED AS:Shazam H, Butt Y.Diagnostic and prognostic role of the SARS-CoV-2-specific IgA antibody: an updated systematic review and meta-analysis. Khyber Med Univ J 2023;15(2):122-33. https://doi.org/10.35845/kmuj.2023.22498 |
ABSTRACT
OBJECTIVE: This systematic review and meta-analysis aimed to investigate the diagnostic accuracy and prognostic role of the SARS-CoV-2-specific IgA antibody.
METHODS: A computerized search was conducted on MEDLINE, Scopus, Web of Science, Cochrane Library, and Google Scholar, using the relative keywords. Received citations were screened, and relevant data were extracted from the included studies. The methodological quality of the articles was assessed through modified version of the quality assessment of diagnostic accuracy studies tool-2. To analyze the risk of bias, four domains were reviewed: patient selection, index test, reference standard and flow & timing; three domains, patient selection, index test, and reference standard, were assessed based on applicability. Review Manager-5.4 and Meta-DiSc were used for analysis.
RESULTS: Twenty-nine studies were included in the qualitative synthesis, and only 15 studies were included in the quantitative synthesis. On pooled analysis, the overall sensitivity of IgA was 90% (87% to 92%), specificity 94% (92% to 95%), positive likelihood ratio (LR) 9.33 (3.40 to 25.57), negative LR 0.14 (0.05 to 0.37), diagnostic Odds Ratio 71.5 (13.22 to 382.80), and overall area under the curve was 96.5%. Overall random effect estimate demonstrated a significant elevation of IgA in the severe group [standardized mean difference=0.23, 95% CI 0.10 to 0.36), p=0.0005], compared to non-severe group.
CONCLUSION: The current evidence suggests that IgA has good sensitivity, specificity, and accuracy in detecting patients with COVID-19 along with the clinical and laboratory characteristics. The prognostic role of IgA needs more comprehensive investigations on larger samples to reach conclusive evidence.
KEYWORDS: Immunoglobin A (MeSH); Sensitivity (MeSH); Specificity (MeSH); Prognosis (MeSH); Diagnosis (MeSH); COVID-19(MeSH).
INTRODUCTION
The prevalence of coronavirus disease 2019 (COVID-19) continues to increase, reaching more than 254 million confirmed cases and 5.11 million deaths globally.1 In order to prevent the spread of the disease, infected patients must be identified and quarantined as soon as possible. Several COVID-19 diagnostic tests have been reported. SARS-CoV-2 virological testing is frequently suggested for the diagnosis of COVID-19 because it offers the most conclusive proof of the virus's existence.2 Reverse transcription-polymerase chain reaction (RT-PCR), the gold standard diagnostic test suggested by current recommendations, may identify SARS-CoV-2 RNA in respiratory samples.3However, a number of variables have been found to significantly impact the performance of RT-PCR tests, including improper specimen collecting procedures, viral load, time since exposure, and specimen source, all of which might lead to false-negative tests findings.4,5 As a result, more diagnostic tests are urgently required to reduce the risk of misdiagnosis or developing critical illness, which may lead to hospitalization and even death.
Serological testing for specific antibodies against SARS-CoV-2, such as immunoglobulin A (IgA), IgG, and IgM antibodies, have been proposed as additional diagnostic techniques since they can reveal information regarding current or past infection.6,7 While some articles revealed that serological tests have a good sensitivity (up to 97.8%) and better accuracy when combined with PCR, there is a scarcity of high-quality evidence supporting the utilization of immunoglobulin screening for COVID-19.8The subtype of antibody, serological test kit, detection duration, and measurement technique all varied considerably between studies.9 There is no agreement on how to interpret antibody test findings. The diagnostic accuracy of immunoglobulins may be affected by the link between their presence and the disease severity or immunization.10 IgA tests, in addition to IgG, might be beneficial in individuals presenting with uncommon symptoms as well as when RT-PCR persistently showed negative results in a suspected case. IgA was the most common immunoglobulin found in early COVID-19 illness, according to Zervou et al.10 Furthermore, they stated that IgA levels were greatest in individuals with a serious and life-threatening condition.10Moreover, Caselli and his colleagues highlighted that IgA detection may be valuable in the detection of COVID-19 and the prediction of its outcomes.11 However, there is a need for developing a systematic review and meta-analysis to summarize and criticize the current evidence. Therefore, the purpose of this meta-analysis was to look at the diagnostic and prognostic role of the SARS-CoV-2-specific IgA antibody.
Research Questions
· Based on the current literature, what is the diagnostic test accuracy of IgA antibody SARS-CoV-2 in patients with COVID-19?
· Based on the current literature, what is the prognostic role of IgA antibody SARS-CoV-2 in patients with COVID-19?
METHODS
Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed while conducting this study.12 PubMed, Scopus, Web of Science, Cochrane Library, and Google Scholar were used to search relevant articles systematically. The search term was as follows: (Immunoglobulin A OR "Immunoglobulin A*" OR IgA OR "IgA Antibody" OR "Antibody, IgA" OR IgA1 OR IgA2) AND (COVID-19 OR "COVID 19" OR SARS-COV-2 OR Coronavirus OR COVID). In addition, we performed a manual search by looking at the references in the included articles and the relevant references in PubMed.
Inclusion and exclusion criteria
Those studies satisfying all of the following criteria were included in the present study:
(1) Population: Studies that included patients with confirmed COVID-19 with RT-PCR, serology, and clinical presentation;
(2) Intervention/Exposure: Studies that assessed the level of IgA against SARS-CoV-2 using RT-PCT or ELISA;
(3) Comparator/Control: Studies that compared between IgA and the gold standard method or IgA and other immunoglobulins;
(4) Outcome: Studies that reported the diagnostic accuracy of IgA and/or the its role in differentiation between mild and severe cases (prognosis);
(5) Study design: Observational (cohort, case-control, or cross-sectional) studies.
Theses, conference abstracts, reviews, non-English studies, and studies whose data were unreliable for extraction and analysis were all excluded.
Study Selection
Two independent reviewers performed two distinct phases of eligibility screening: (a) titles and abstract screening and (b) full-text screening. Disagreements between the two reviewers were addressed through conversation.
Data Extraction
Using an offline data extraction sheet, two authors independently extracted the data. (1) study design and ID; (2) patient’s characteristics; (3) risk of bias domains; and (4) outcomes.The census was used to resolve disagreements.
Risk of bias assessment
The methodological quality of the articles was assessed using a modified version of the quality assessment of diagnostic accuracy studies tool-2 (QUADAS-2).13To analyze the risk of bias, four domains were reviewed: patient selection, index test, reference standard, and flow and timing; three domains, namely patient selection, index test, and reference standard, were assessed based on applicability.
Data Synthesis
Extracted data were analyzed using Review Manager 5.4 and Meta-DiSc 1.4 software. To detect the diagnostic value of IgA, we performed a diagnostic test accuracy analysis using the extracted data of each study, including the numbers of included patients, true-positive (TP), true-negative (TN), false-positive (FP), and false-negative (FN). The test was performed to identify the sensitivity, specificity, positive likelihood ratio (LR), negative LR, and Summary receiver operating characteristics (SROC) curve. To identify the prognostic value of IgA, we used the contentious comparison between the severe and non-severe COVID-19 cases using the Inverse-variance (I-V) method with a Random-effect estimate to generate the standardized mean difference (SMD) between both groups. Heterogeneity was resolved with a sensitivity analysis.
RESULTS
Literature search results and Characteristic of included studies
Our search of the databases resulted in 3260 articles, which were reduced to 2760 after removing the duplications. About 2600 studies were excluded in the stage of Title/Abstract screening, and 131 studies were excluded in the stage of full-text screening, resulting in 29 included studies in the qualitative synthesis (Systematic review). Out of them, only 15 articles were included in the Meta-analysis. The PRISMA flow diagram is presented in Figure 1.
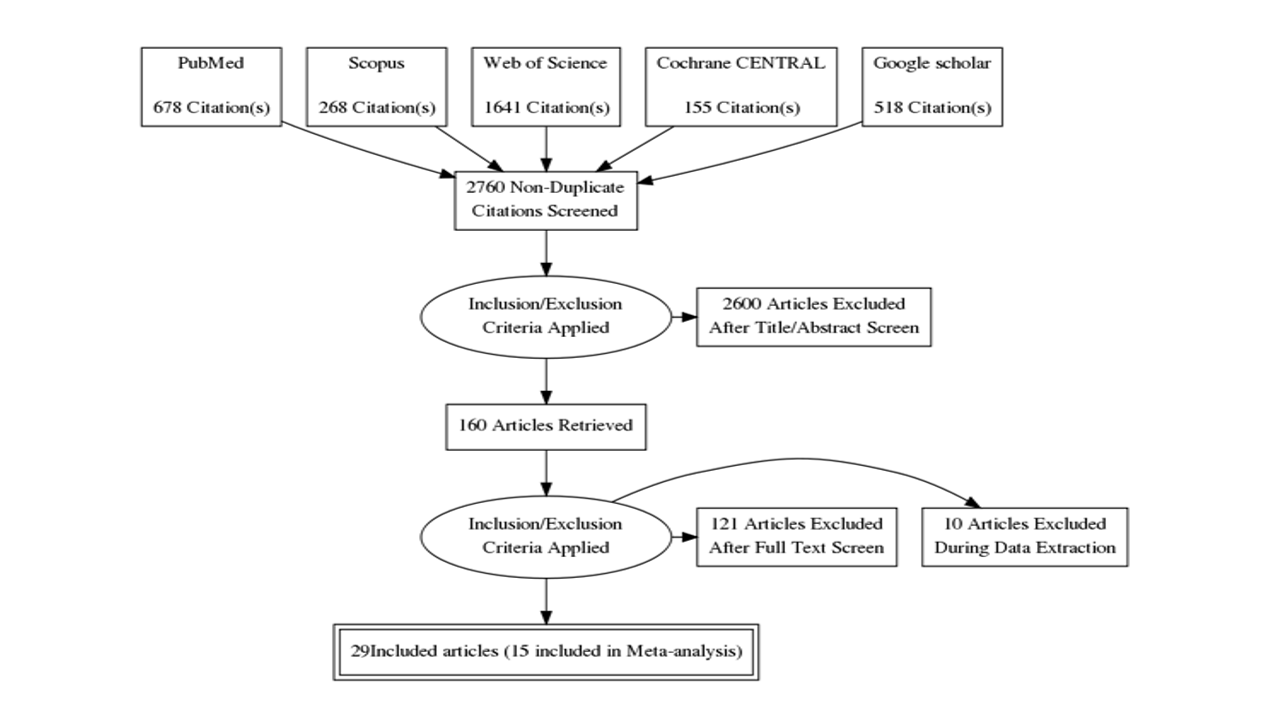
Figure1: PRISMA flow diagram of the study
Out of the included studies, 19 studies were cohort and 10 studies were cross-sectional. In terms of method of IgA detection, 15 studies used ELISA alone, six studies used RT-PCR alone, and five studies used both ELISA and RT-PCR, one study used Chemiluminescence method, one study used Lateral flow immunochromatographic (LFIA) and electro-chemiluminescence immunoassay (ECLIA), and one study did not report the used method (Table I).
Table I: Summary table of baseline characteristics of the studies included
|
Study ID |
Country |
Study design |
Method of IgA detection |
Time from symptom onset to sampling |
Main findings |
|
Zervou 202110 |
USA
|
Cohort study |
ELISA |
-For 82 hospitalized patients: between 1-59 days
- For 100 ambulatory hospital employees: between 35-57 days |
-IgA was detected in 60% of samples within 7 days’ post onset of symptoms. -Patients with severe and critical illness showed the highest IgA levels. - Regardless of age, sex, and duration of symptoms, there was a statistically significant correlation between IgA levels and critical disease (p = .05). - IgA was detected only in 10% of 100 ambulatory hospital employees who had antibody testing after 28 days post onset of symptoms |
|
Reinwald 202114 |
Germany |
Cohort study |
ELISA |
NR |
-At follow up period, seroprevalence of anti-SARS-CoV-2 IgA was increased from 11.7% to 15%. - There was a correlation between symptom burden increase and class switch from IgA to IgG |
|
Xue 202115 |
China |
Cohort study |
Chemiluminescence method |
The average time was 8 days |
-The positive rate of IgA was 97% in the cohort. -A peak of IgA was detected after admission by 10-15 days. - The progress of pulmonary lesions in severe COVID-19 patients can be predicted by combination of IgA and IgG and this combination is closely related to hypoxemia - It was found that IgA and IgG have an important role in the defense against destruction and invasion of bronchial and alveolar epithelium by the virus. |
|
Staats 202016 |
Germany |
Cohort study |
ELISA |
NR |
-There was a restriction of IgA2 to severe disease -In severe cases, IgA2 showed the strongest discrimination between nonfatal and fatal outcome. -There was a correlation between CRP levels and anti-SARS-CoV-2 IgG and IgA2 levels in severe cases - There was a correlation between anti-SARS-CoV-2 IgA2 and ecDNA. |
|
Aita 202017 |
Italy |
Cohort study |
ELISA |
NR |
- Saliva and nasopharynx swabs were both negative in 35 (82%) out of 43 patients while there were SARS-COV-2 positive in 7 (16%). - Saliva and NP swabs did not match perfectly in one case (Negative NP-swab, Positive saliva). - Positive molecular results had been significantly correlated with the duration of the disease (p = 0.0049). - SARS-COV-2 was negative on both saliva and NP-swab in 326/326 screened subjects. - 18 out of 27 saliva samples tested for IgA had been found to be positive. - Positivity of salivary IgA had been associated with CPR values (p = 0.0183) and pneumonia (P= 0.002), not with other molecular and clinical data or other serum immunoglobulins. |
|
Carnicelli 202118 |
Italy
|
Cross- sectional |
ELISA |
23 (12-45) days (Median-IQR) |
- The analysis revealed that IgA seroconversion seems to happen between 6 and 15 days. - Severe cases demonstrated higher response of IgA in comparison with mild cases when analyzing optical density (8.3 versus 5.6, p < 0.001). - After the stratification according to the disease severity, IgA response had been found to be more vigorous in severe cases. |
|
Caramelli 202119 |
Brazil |
Cross-sectional |
ELISA |
NR |
- This study found that there was no difference in the prevalence of IgA and IgG positive test between women and men and in individuals over 60 years and under 60 years of age. - Controversy, analyzing only patients with positive samples showed that IgG levels in positive samples were higher than positive IgA ones, 3.00 (IQR = 1.68-5.65), and 1.95 (IQR = 1.40-3.38), respectively; P = 0.01 7 - Moreover, patients with isolated IgA positive samples had significantly lower IgA levels than those with positive tests of IgG and IgA 1.95 (IQR = 1.60-2.40) and 3.15 (IQR = 2.20-3.90), respectively, P = 0.005 - These data reported in this study demonstrated that IgA distribution showed a deviation to the left when compared with IgG distribution data. Furthermore, many cases with positive IgA had slightly elevated levels of immunoglobulin. |
|
Caselli 202011 |
Italy |
Cross-sectional |
ELISA |
Between 1-78 days after hospitalization |
- The results revealed that 35.7% of SARS-COV-2 patients have particular antiviral IgA at the ocular level that lasts up to two days post the onset of the disease. - Most of IgA positive cases showed mild symptoms. - The study data demonstrated that the anti-SARS-COV-2 IgA had long persistence at the eye level and suggested that the detection of IgA can be immensely helpful for the clarification of the virus epidemiology and pathology. |
|
Fox 202020 |
USA |
Cross-sectional |
ELISA |
3-4 days |
- All samples showed significant reactivity of specific IgA to the full spike, while 80% showed that secretory Ab and specific IgA are binding to Receptor-Binding Domain. - IgA was highly correlated with sAb which indicated that most IgA to be sIgA. - Overall, the study data showed that in the human milk, the response of a robust sIgA-dominant SARS-CoV-2 Ab should be anticipated in a substantial majority of patients. |
|
Ali 202021 |
Switzerland |
Cohort study |
Lateral flow immunochromatographic (LFIA) and electro-chemiluminescence immunoassay (ECLIA) |
Two weeks |
- The increased total IgA was significantly associated with severe illness (sdCOVID, P=0.01; scCOVID, p-value<0.001). - Among antiphospholipid antibodies, both cohorts were severe illness significantly correlated with anti-Beta2 Glycoprotein-1 IgA (sdCOVID and scCOVID, P<0.001), and high anti-Cardiolipin IgA (sdCOVID and scCOVID, p-value<0.001). |
|
Infantino 202022 |
Italy |
Cross-sectional |
ELISA |
5-7 days |
- Eight patients out of 30 patients included in this study were positive for IgA at the Time from symptom onset. -This study recommended the inclusion of IgA antibodies among COVID-19 serological test. |
|
Ivanov 202123 |
Russia |
Cross-sectional |
ELISA |
NR |
- The study showed that about 39.22% of all included COVID-19 patients showed unusual change in the levels of plasma anti-SARS-CoV-2 IgA. - IgA high levels persisted for more than six months after recovery. - IgA antibodies showed more robust response to COVID-19 and appeared earlier than IgG. - Throughout the observation period, the increased anti-SARS-CoV-2 IgA levels had been recorded in 28 out of 180 participants, one of them was infected with COVID-19 disease. |
|
Juncker 202124 |
Netherlands |
Cohort study |
ELISA |
Average of 8 weeks after PCR confirmation |
- Of 691 participants, 524 had IgA against COVID-19 in the human milk. - This study showed that specific immunoglobulin A persisted in the human milk for about ten months after a polymerase chain reaction which confirms the COVID-19 infection. |
|
Needle 202125 |
Canada |
Cross-sectional |
ELISA |
60 days (38-67) Median (IQR) |
- This study revealed that the sensitivity of EuroImmun SARS-CoV-2 ELISA immunoglobulin class A (IgA) was 91.3% while the IgA specificity was 90.8%. |
|
Cao 202026 |
China |
Cohort study |
NR |
2 days |
ICU-patients had an increased level of IgA compared with non ICU-patients (21.1% vs 7.4%) |
|
Chen 202027 |
China |
Cohort study |
Real time PCR |
16 (12.0-20.0) (Median time from disease onset to death) |
There was no significant difference in the serum circulating IgA between the recovered group and death group. |
|
Fu 202028 |
China |
Cohort study |
Real time PCR |
NR |
There was no significant difference in the serum circulating IgA between the survival group and death group. |
|
He 202029 |
China |
Cohort study |
Real time PCR |
NR |
There was no significant difference in the IgA level between the severe group and non-severe group. |
|
Han 202030 |
China |
Cohort study |
Real time PCR |
NR |
There was no significant difference in the IgA level between the severe and non-severe groups. |
|
Liu 2020 31 |
China |
Cohort study |
Real time PCR |
NR |
There was no significant difference in the IgA level between the severe and mild groups. |
|
Qin 202032 |
China |
Cohort study |
Real time PCR |
NR |
There was no significant difference in the IgA level between the severe and mild groups. The level of IgA in COVID-19 patients was within the normal range. |
|
Beavis 202033 |
USA |
Cross-sectional |
ELISA |
NR |
Of 82 samples from SARS-COV-2 PCR-positive cases, 68 tested positive a14 tested negative for IgA. The real-time PCR assay showed good specificity for IgA. |
|
Brandstetter 202034 |
Germany |
Cross-sectional |
ELISA |
NR |
About 3 weeks after the onset of COVID-19 symptoms, 80% of the COVID-19 cases developed some specific IgA responses. Also, subjects in the non-COVID group had an elevated IgA values (7.6%). |
|
Jääskeläinen 202035 |
Finland |
Cross-sectional |
ELISA |
11 days |
IgA specificity was 73.0%. Of 39 COVID-19 patients, 11 were IgA positive |
|
Lassaunière 202036 |
Denmark |
Cohort study |
ELISA, PCR |
|
The results showed that the specificity of the IgA was 93% while the sensitivity was 90%. |
|
Montesinos 202037 |
Belgium |
Cohort study |
ELISA, PCR |
10 days |
Euroimmun IgG/IgA test showed higher sensitivity than Maglumi™ IgG/IgM test (64.3 % Versus 84.4 %) |
|
Colkesen 202138 |
Turkey |
Cohort study |
ELISA, PCR |
NR |
Serum IgA was significantly higher in the severe group compared to mild group |
|
Ma 202039 |
China |
Cohort study |
ELISA, PCR |
4–10 |
IgA levels in severe cases were significantly higher than those mild or moderate cases. IgA detection shows the highest sensitivity during about 4–25 days after illness onset |
|
Nie 202040 |
China |
Cohort study |
ELISA, PCR |
NR |
IgA levels in severe cases were comparable to those of mild cases. |
NR: Not reported
Regarding the included patients, the overall number was 7291 patients and 126 controls. The age of the included patients ranged from 30 months to over 80 years old. In terms of the methods used of COVID-19 diagnosis, there was a huge variety between the included studies, including RT-PCR (most common), serology, and clinical presentation.
Risk of bias and applicability
Regarding the risk of bias, only three studies have high risk of bias in term of patient selection (10.34%), five studies in index test (17.24%), three studies in reference standards (10.34%), and 11 studies in flow and timing (37.93%). No studies have applicability concerns. The summary of risk of bias and applicability is presented in Figure 2.
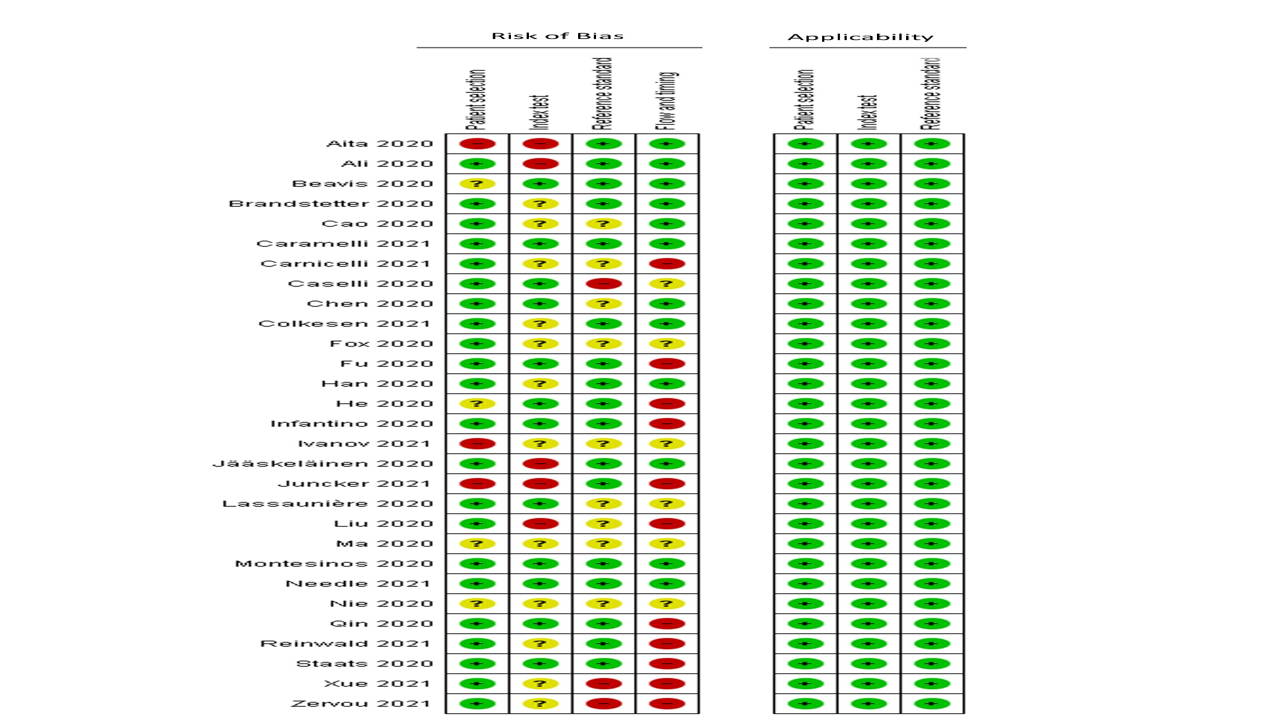
Figure 2: Risk of bias summa
Diagnostic test accuracy of IgA
Six studies reported data regarding the diagnostic accuracy of IgA in patients with confirmed COVID-19 using the gold-standard test (RT-PCR). The pooled analysis showed that the overall sensitivity of IgA was 90% (87% to 92%) Figure 3, and the overall specificity was 94% (92% to 95%), Figure 4.
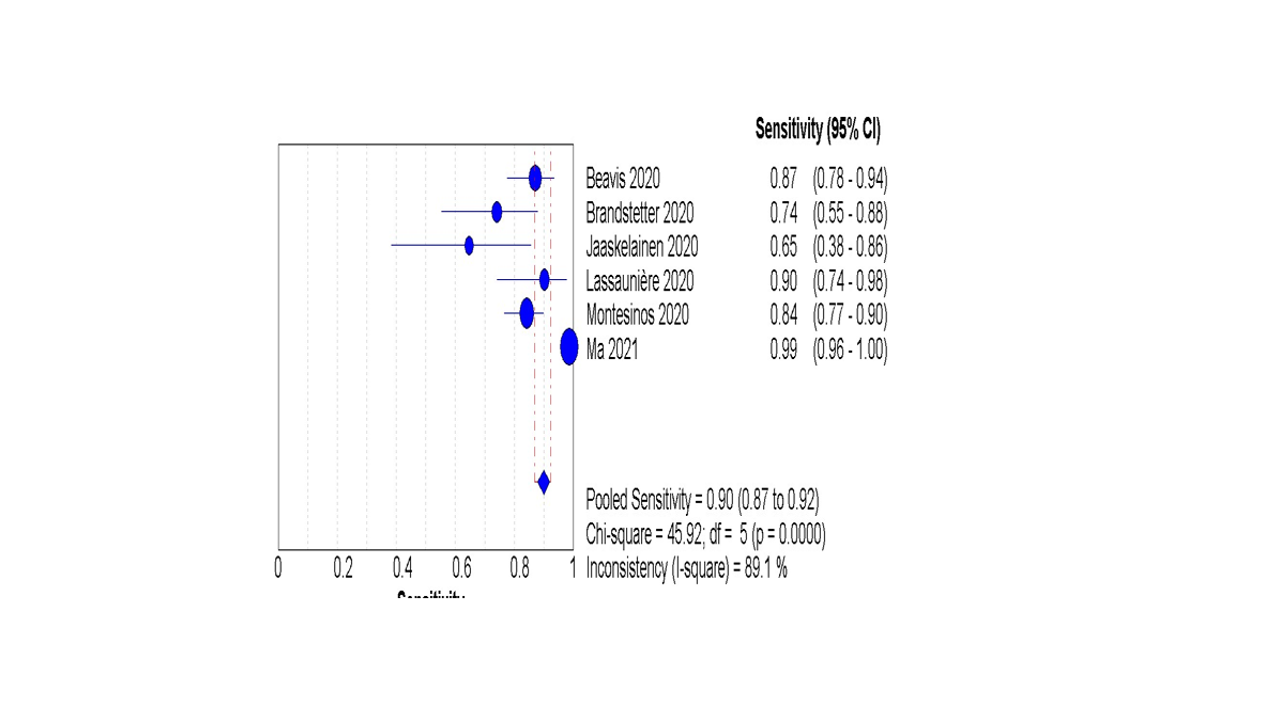
Figure 3: The pooled analysis of the IgA sensitivity
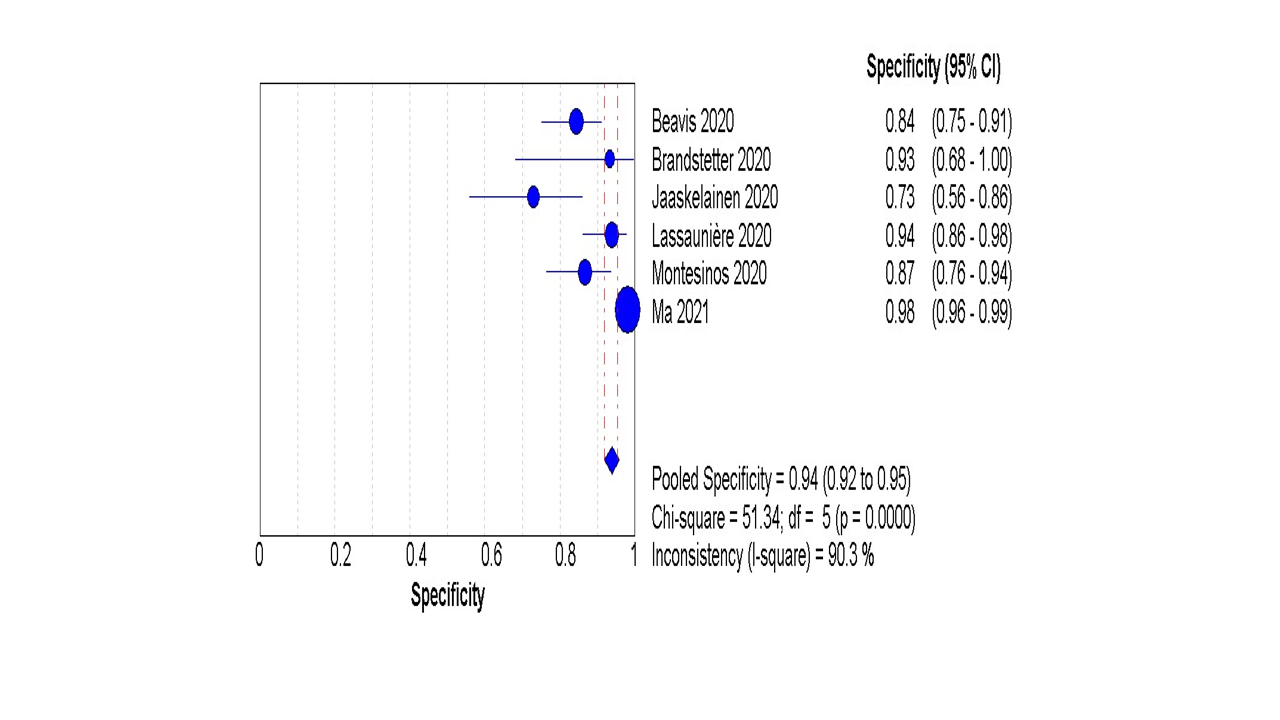
Figure 4: The pooled analysis of the IgA specificity
The random effect estimate of +ve LR was 9.33 (3.40 to 25.57), and the –ve LR was 0.14 (0.05 to 0.37) S1 F1A and S1 F1B. The pooled diagnostic OR was 71.5 (13.22 to 382.80) S1 F2. Furthermore, the pooled specificity was 94% (92% to 95%). The overall AUC was 96.5%, Figure 5.
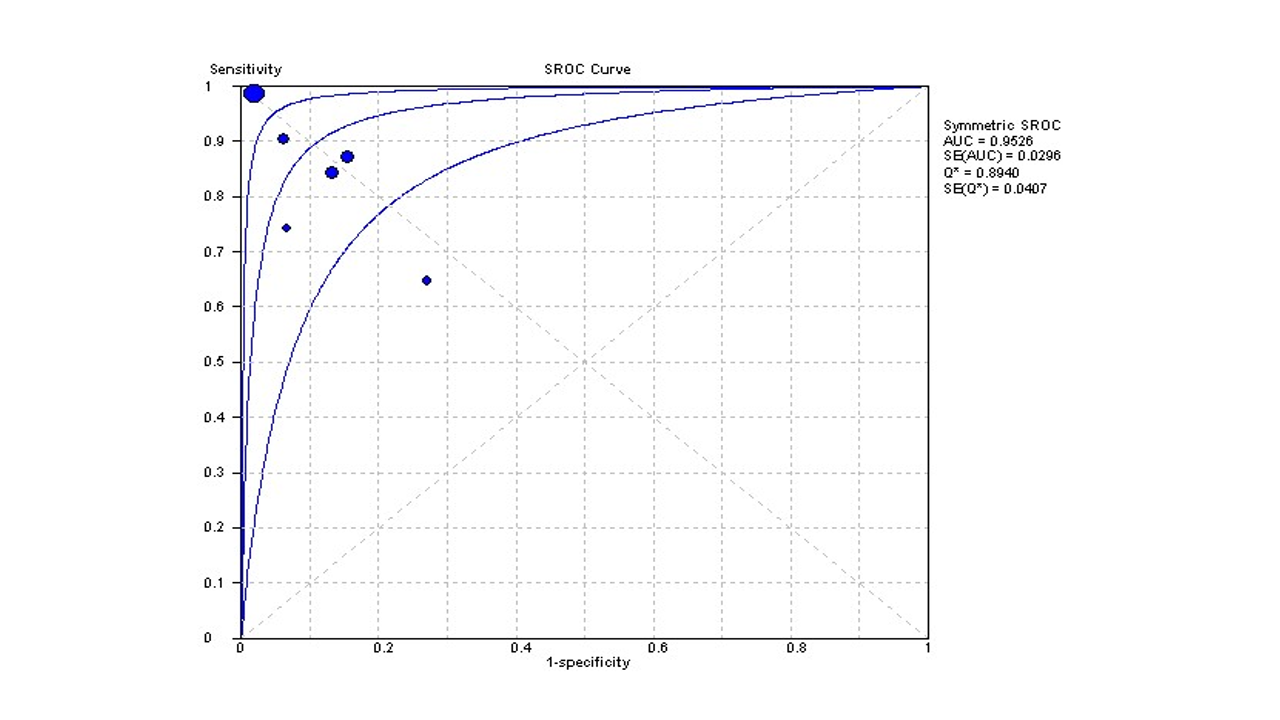
Figure 5: Summary receiver operating characteristics (sROC) curve
Prognostic value
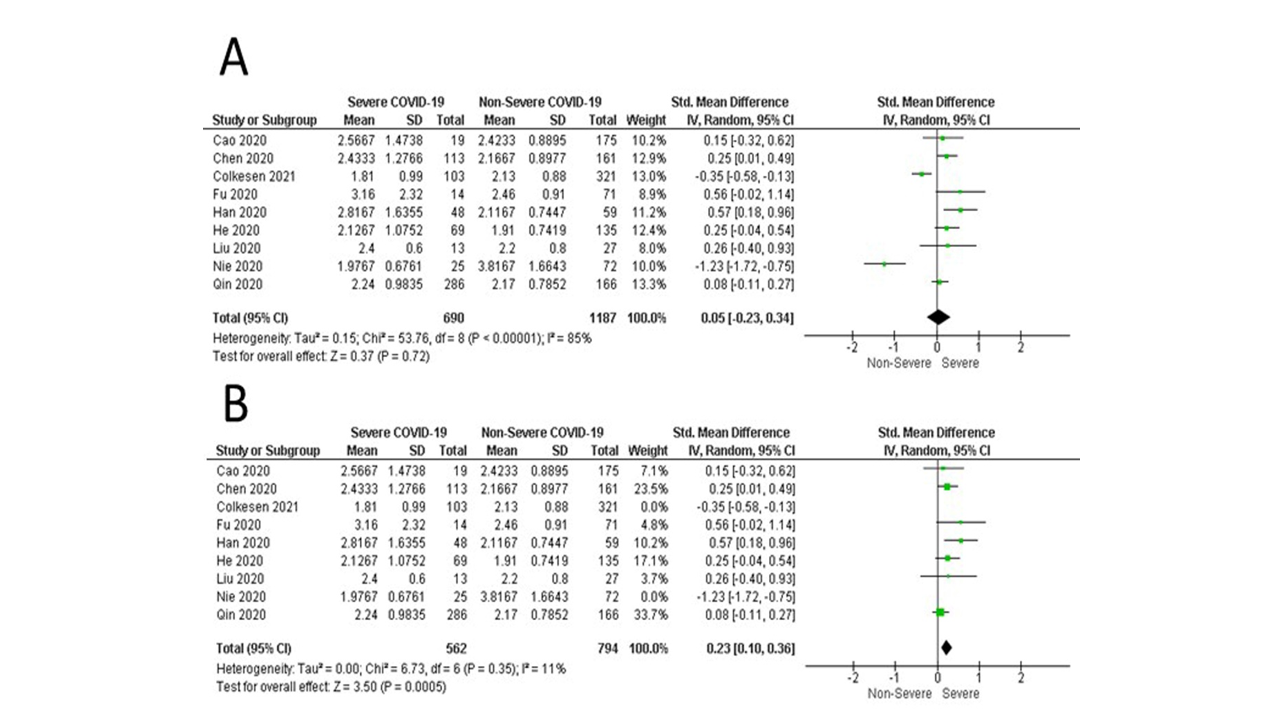
Nine studies compared between the severe cases of COVID-19 and non-severe cases in terms of IgA profile. The overall random effect estimate demonstrated that both severe and non-severe cases have a comparable IgA profile with a mean SMD [0.05, 95% CI (-0.23 to 0.34), p=0.72]. Pooled data were heterogeneous (I2= 85%; p<0.00001). Sensitivity analysis was applied to detect the source of heterogeneity. After excluding the Colkensen 2021 study and Nie 2020 study, the pooled data were homogenous (I2= 11%; p=0.35), with a significant elevation of IgA in the severe group [SMD= 0.23, 95% CI (0.10 to 0.36), p=0.0005] (Figure 6).
Figure 6: A) The pooled analysis of the standardized mean difference of IgA between severe and non-severe cases of COVID-19; B) The pooled analysis of the standardized mean difference of IgA between severe and non-severe cases of COVID-19 after applying the sensitivity ananlysisby excludingColkesen et al., 2021 and Nie et al., 2020
DISCUSSION
In this systematic review and meta-analysis, we have evaluated the diagnostic and prognostic value of IgA in patients with COVID-19. Our findings showed that IgA has a significant role in detecting SARS-CoV-2 as it has a relatively high sensitivity, specificity, and accuracy. In terms of its role in SARS-CoV-2 prognosis, due to the significant heterogeneity, the current evidence cannot be used as conclusive evidence; however, patients with severe cases of COVID-19 were associated with higher levels of IgA compared with the non-severe patients.
The SARS-CoV-2 virus enters human cells through ACE2 receptors, which are located on alveolar epithelial cells.28The virus's spike protein can be recognized by the RBD, and the body's initial response is to generate IgM antibodies to fight the virus.41,42These antibodies can be detected in the blood as early as the third day of symptomatic infection. Later, the body switches to producing IgG antibodies, or in some cases, IgA antibodies which are produced in the lamina propria by plasma cells.43,44 Research on IgA production in COVID-19 patients is limited and most studies focus on IgM, IgG, and total immunoglobulins.10 IgA plays an important role in mucosal immunity, specifically as an immunological barrier that neutralizes SARS-CoV-2 before it reaches and binds to epithelial cells. Measuring the quantity of RBD-specific IgA in the respiratory mucosa could serve as a marker of the host's immunological response, and this can be directly quantified in saliva and tears, potentially making IgA detection an early diagnostic marker for COVID-19.45
In the study of Lassaunière et al., the authors showed that IgA ELISA had 93% specificity, compared to 96% for IgG. However, the sensitivity of the IgA ELISA was much better than IgG ELISA (93% vs. 67%), respectively. On the other hand, they highlighted that both IgA and IgG are also more prone to cross-react with negative sera, which may affect the accuracy of both tests.36Similarly, Jaaskelainen and his colleagues reported that IgG ELISA had a much better specificity than IgA ELISA (91.9% vs. 73%) respectively; therefore, they did not recommend using IgA in the initial screening. Nevertheless, they stated that using IgA along with IgG in the acute phase could be helpful in a suspected case with repeatedly negative RT-PCR.The cross-reaction was observed in one patient with HCoV OC43 infection; however, there was no reaction in cases of HCoV229E or NL63 infections. In addition, they found that the median for IgA alone was 11 days (5-20 days). 35On the other hand, Montesinos et al., reported that IgA ELISA was more sensitive (83.6%) than IgG (61.7%) but less specific (86.1% vs. 98.6%), respectively. They concluded that both IgA and IgG demonstrated high accuracy and equivalent performance of the other CLIA tests in spotting SARS-CoV-2 antibodies 14 days after the onset of COVID-19 symptoms.37 In the study of Beavis et al., IgA showed good sensitivity and specificity in samples collected four days after detecting COVID-19 with RT-PCR. Some scientists have suggested that IgA plays a more important role in individuals who are not severely impacted by SARS-CoV-2 infection, although evidence on the subject is still inconclusive.33
Regarding the prognostic value of IgA, Cao et al., showed that patients admitted to the ICU were more likely to have an increased level of IgA (21.1% vs. 7.4%).26 Also, He et al., mentioned that patients with comorbidities were associated with a significantly higher IgA, IgM, and C4, compared with patients with no comorbidities.24 On the other hand, Han and his team demonstrated that there was no significant difference between severe and non-severe patients in terms of IgA level (p=0.053).29 Likewise, Qin et al., could not find any significant differences between mild and severe cases regarding the levels of IgA, IgG, and complement proteins C3 or C4, while IgM slightly decreased in severe ones.32The same was observed by Liu et al.; however, they found that IgM was comparable in both groups. 31 Compared to the survived group, the death group had a comparable IgA, IgG, IgM, IgE, C3, and C4, according to the study. 46However, after pooling all of these findings, and after removing the heterogenous studies, our findings showed that patients with severe COVID-19 are associated with higher levels of IgA compared to the non-severe group. Severe COVID-19 is linked with increased total IgA and IgA antiphospholipid antibodies, according to Ali and his colleagues. These findings imply that a strong IgA response to SARS-CoV-2 might lead to systemic autoimmunity.21According to Caramelli et al., being IgA positive is not enough to implement the recommendations of COVID-19; the patient should undergo a comprehensive evaluation of clinical and radiological parameters, as they found that many positive patients have slightly low IgA levels, which is less than the cut-off point value. 19
Chen et al., conducted a meta-analysis to evaluate the diagnostic test accuracy of all SARS-CoV-2 specific antibodies. They have included 68 studies. The pooled analysis showed that the sensitivity of IgG, IgA, IgM+, IgG+IgM+/−, IgM+IgG+/−, IgM+IgG+, IgG+IgM−, and IgM+IgG− was 79%, 78%, 73%, 68%, 53%, 7%, and 6%, respectively. Moreover, the specificity of all antibodies ranged from 98% to 100%, except for IgA, which has a specificity of 88%. In addition, they observed that performing the test two weeks after the symptomatic infection was associated with better results compared with earlier test. Therefore, they concluded that the capacity of these antibodies to diagnose COVID-19 early could be limited. 27
Another meta-analysis tied to find the predictors of COVID-19 severity. They included 149 studies; however, only eight studies were included about IgA. IgA and IgG antibodies to SARSCoV2 were substantially higher in the severe group than in the non-severe group, whereas IgM antibodies were somewhat lower in the severe patients than in the non-severe patients, and IgE antibodies revealed no significant intergroup differences. 47
We acknowledge that our study has some limitations. First, the number of included studies and included patients is considerably low. However, we included only the studies that answered our specific question, and the number of published studies about IgA and COVID-19 is low. Second, the quality of included studies cannot be considered high; however, the quality of published studies in the era of COVID-19 witnessed a significant reduction due to the rush to publish. Third, the detected heterogeneity was high, but we solved it with performing a sensitivity analysis. Fourth, we could not perform subgroup analysis due to lack of required data.
CONCLUSION
The current evidence suggests that IgA has good sensitivity, specificity, and accuracy in detecting patients with COVID-19 along with the clinical and laboratory characteristics. The prognostic role of IgA needs more comprehensive investigations with larger samples to reach conclusive evidence.
REFERENCES
1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19(3):141-54. https://doi.org/10.1038/s41579-020-00459-7
2. Emery SL, Erdman DD, Bowen MD, Newton BR, Winchell JM, Meyer RF, et al. Real-Time Reverse Transcription–Polymerase Chain Reaction Assay for SARS-associated Coronavirus. Emerg Infect Dis 2004;10(2):311-6. https://doi.org/10.3201/eid1002.030759
3. Kanji JN, Zelyas N, MacDonald C, Pabbaraju K, Khan MN, Prasad A, et al. False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol J 2021;18(1):13. https://doi.org/10.1186/s12985-021-01489-0
4. De Pace V, Caligiuri P, Ricucci V, Nigro N, Galano B, Visconti V, et al. Rapid diagnosis of SARS-CoV-2 pneumonia on lower respiratory tract specimens. BMC Infect Dis 2021;21(1):926. https://doi.org/10.1186/s12879-021-06591-w
5. Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020;323(22):2249-51. https://doi.org/10.1001/jama.2020.8259
6. Nakano Y, Kurano M, Morita Y, Shimura T, Yokoyama R, Qian C, et al. Time course of the sensitivity and specificity of anti-SARS-CoV-2 IgM and IgG antibodies for symptomatic COVID-19 in Japan. Sci Rep 2021;11(1):2776. https://doi.org/10.1038/s41598-021-82428-5
7. Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ 2020;m2516. https://doi.org/10.1136/bmj.m2516
8. Wuthiekanun V, Amornchai P, Chierakul W, Cheng AC, White NJ, Peacock SJ, et al. Evaluation of Immunoglobulin M (IgM) and IgG Rapid Cassette Test Kits for Diagnosis of Melioidosis in an Area of Endemicity. J Clin Microbiol 2004;42(8):3435-7. https://doi.org/10.1128/jcm.42.8.3435-3437.2004
9. Chao YX, Rötzschke O, Tan EK. The role of IgA in COVID-19. Brain Behav Immun 2020;87:182-3. https://doi.org/10.1016/j.bbi.2020.05.057
10. Zervou FN, Louie P, Stachel A, Zacharioudakis IM, Ortiz‐Mendez Y, Thomas K, et al. SARS‐CoV‐2 antibodies: IgA correlates with severity of disease in early COVID‐19 infection. J Med Virol 2021;93(9):5409-15. https://doi.org/10.1002/jmv.27058
11. Caselli E, Soffritti I, Lamberti G, D’Accolti M, Franco F, Demaria D, et al. Anti-SARS-Cov-2 IgA Response in Tears of COVID-19 Patients. Biology (Basel) 2020;9(11):374.https://doi.org/10.3390/biology9110374
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
13. Whiting PF. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med 2011;155(8):529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
14. Reinwald M, Deckert PM, Ritter O, Andresen H, Schreyer AG, Weylandt KH, et al. Prevalence and Course of IgA and IgG Antibodies against SARS-CoV-2 in HealthcareWorkers during the First Wave of the COVID-19 Outbreak in Germany: Interim Results from an Ongoing Observational Cohort Study. Healthcare (Basel) 2021;9(5):498. https://doi.org/10.3390/healthcare9050498
15. Xue M, Zhang T, Hu H, Huang Z, Zhen Y, Liang Y, et al. Predictive effects of IgA and IgG combination to assess pulmonary exudationprogression in COVID-19 patients. J Med Virol 2021;93(3):1443-8. https://doi.org/10.1002/jmv.26437
16. Staats LAN, Pfeiffer H, Knopf J, Lindemann A, Fürst J, Kremer AE, et al. IgA2 antibodies against SARS-CoV-2 correlate with NET formation and fatal outcomein severely diseased COVID-19 patients. Cells 2020;9(12):2676. https://doi.org/10.3390/cells9122676
17. Aita A, Basso D, Cattelan AM, Fioretto P, Navaglia F, Barbaro F, et al. SARS-CoV-2 identification and IgA antibodies in saliva: One sample two testsapproach for diagnosis. Clin Chim Acta 2020;510:717-22. https://doi.org/10.1016/j.cca.2020.09.018
18. Carnicelli A, Fiori B, Ricci R, Piano A, Bonadia N, Taddei E, et al. Characteristic of IgA and IgG antibody response to SARS-CoV-2 infection in anItalian referral COVID-19 Hospital. Intern Emerg Med 2022;17(1):53-64. https://doi.org/10.1007/s11739-021-02750-8
19. Caramelli B, Escalante-Rojas MC, Chauhan HKC, Siciliano RF, Bittencourt MS, Micelli AC. The “false-positive” conundrum: IgA reference level overestimates theseroprevalence of antibodies to SARS-CoV-2. J Glob Health 2021;11:05001. https://doi.org/10.7189/jogh.11.05001
20. Fox A, Marino J, Amanat F, Krammer F, Hahn-Holbrook J, Zolla-Pazner S, et al. Robust and Specific Secretory IgA Against SARS-CoV-2 Detected in Human Milk. iScience 2020;23(11):101735. https://doi.org/10.1016/j.isci.2020.101735
21. Ali OH, Bomze D, Risch L, Brugger SD, Paprotny M, Weber M, et al. Severe Coronavirus Disease 2019 (COVID-19) is Associated With Elevated Serum Immunoglobulin (Ig) A and Antiphospholipid IgA Antibodies. Clin Infect Dis 2021;73(9):e2869-74. https://doi.org/10.1093/cid/ciaa1496 Erratum in: Clin Infect Dis 2021;73(9):1746. https://doi.org/10.1093/cid/ciab532
22. Infantino M, Manfredi M, Grossi V, Lari B, Fabbri S, Benucci M, et al. Closing the serological gap in the diagnostic testing for COVID-19: The value ofanti-SARS-CoV-2 IgA antibodies. J Med Virol 2021;93(3):1436-42. https://doi.org/10.1002/jmv.26422
23. Ivanov A, Semenova E. Long-term monitoring of the development and extinction of IgA and IgG responsesto SARS-CoV-2 infection. J Med Virol 2021;93(10):5953-60. https://doi.org/10.1002/jmv.27166
24. Juncker HG, Romijn M, Loth VN, Ruhé EJM, Bakker S, Kleinendorst S, et al. Antibodies against SARS-CoV-2 in human milk: milk conversion rates in theNetherlands. J Hum Lact 2021;37(3):469-76. https://doi.org/10.1177/08903344211018185
25. Needle R, Gilbert L, Zahariadis G, Yu Y, Dalton-Kenny H, Russell RS, et al. Serological Evaluation of human antibodies of the immunoglobulin class A and Gagainst SARS-CoV-2 in serum collected in Newfoundland and Labrador. Viral Immunol 2021;34(3):182-9. https://doi.org/10.1089/vim.2020.0199
26. Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, et al. Clinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19)in Shanghai, China. medRxiv2020;2020.03.04.20030395. https://doi.org/10.1101/2020.03.04.20030395
27. Chen M, Qin R, Jiang M, Yang Z, Wen W, Li J. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int J Infect Dis 2021;104:415-22. https://doi.org/10.1016/j.ijid.2021.01.016
28. Fu Y, Pan Y, Li Z, Li Y. The Utility of Specific Antibodies Against SARS-CoV-2 in Laboratory Diagnosis. Front Microbiol 2021;11:603058. https://doi.org/10.3389/fmicb.2020.603058
29. He Z, Ren L, Yang J, Guo L, Feng L, Ma C, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies inWuhan, China: a longitudinal, population-level, cross-sectional study. Lancet 2021;397(10279):1075-84. https://doi.org/10.1016/s0140-6736(21)00238-5
30. Han Y, Zhang H, Mu S, Wei W, Jin C, Tong C, et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) 2020;12(12):11245-58. https://doi.org/10.18632/aging.103372
31. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55:102763. https://doi.org/10.1016/j.ebiom.2020.102763
32. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020;71(15):762-8. https://doi.org/10.1093/cid/ciaa248
33. Beavis KG, Matushek SM, Abeleda APF, Bethel C, Hunt C, Gillen S, et al. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA andIgG antibodies. J Clin Virol2020;129:104468. https://doi.org/10.1016/j.jcv.2020.104468
34. Brandstetter S, Roth S, Harner S, Buntrock-Döpke H, Toncheva AA, Borchers N, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2outbreak. Pediatr Allergy Immunol 2020;31(7):841-7. https://doi.org/10.1111/pai.13278
35. Jääskeläinen AJ, Kekäläinen E, Kallio-Kokko H, Mannonen L, Kortela E, Vapalahti O, et al. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro Surveill 2020;25(18):2000603.https://doi.org/10.2807/1560-7917.es.2020.25.18.2000603
36. Lassaunière R, Frische A, Harboe ZB, Nielsen ACY, Fomsgaard A, Krogfelt KA, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv 2020;2020.04.09.20056325. https://doi.org/10.1101/2020.04.09.20056325
37. Montesinos I, Gruson D, Kabamba B, Dahma H, Van den Wijngaert S, Reza S, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol 2020;128:104413. https://doi.org/10.1016/j.jcv.2020.104413
38. Çölkesen F, Kepenek Kurt E, Vatansev H, Korkmaz C, Çölkesen F, Yücel F, et al. Memory B cells and serum immunoglobulins are associated with disease severity andmortality in patients with COVID-19. Postgrad Med J 2022;98(1164):765-71. https://doi.org/10.1136/postgradmedj-2021-140540
39. Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol 2020;17(7):773-5. https://doi.org/10.1038/s41423-020-0474-z
40. Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-basedassay. Nat Protoc 2020;15(11):3699-715. https://doi.org/10.1038/s41596-020-0394-5
41. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46(4):586-90. https://doi.org/10.1007/s00134-020-05985-9
42. Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W, et al. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun 2020;11(1):4541. https://doi.org/10.1038/s41467-020-18319-6
43. Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 2020;11(1):4704. https://doi.org/10.1038/s41467-020-18450-4
44. Klumpp-Thomas C, Kalish H, Drew M, Hunsberger S, Snead K, Fay MP, et al. Standardization of ELISA protocols for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. Nat Commun 2021;12(1):113. https://doi.org/10.1038/s41467-020-20383-x
45. Varadhachary A, Chatterjee D, Garza J, Garr RP, Foley C, Letkeman AF, et al. Salivary anti-SARS-CoV-2 IgA as an accessible biomarker of mucosal immunityagainst COVID-19. medRxiv 2020;2020.08.07.20170258. https://doi.org/10.1101/2020.08.07.20170258
46. Fu YQ, Sun YL, Lu SW, Yang Y, Wang Y, Xu F. Effect of blood analysis and immune function on the prognosis of patients with COVID-19. PLoS One 2020;15(10):e0240751. https://doi.org/10.1371/journal.pone.0240751
47. Liu K, Yang T, Peng X, Lv S, Ye X, Zhao T, et al. A systematic meta‐analysis of immune signatures in patients with COVID‐19. Rev Med Virol 2021;31(4):e2195. https://doi.org/10.1002/rmv.2195
|
Following authors have made substantial contributions to the manuscript as under:
HS:Conception and study design,acquisition, analysis and interpretation of data, drafting the manuscript, critical review, approval of the final version to be published YB:Analysis and interpretation of data, drafting the manuscript, critical review, approval of the final version to be published
Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. |
|
CONFLICT OF INTEREST Authors declared no conflict of interest GRANT SUPPORT AND FINANCIAL DISCLOSURE Authors declared no specific grant for this research from any funding agency in the public, commercial or non-profit sectors |
|
DATA SHARING STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request |
|
|
|
KMUJ web address: www.kmuj.kmu.edu.pk Email address: kmuj@kmu.edu.pk |